Acetaminophen (rectal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acetaminophen (rectal) is an analgesic and antipyretic that is FDA approved for the treatment of minor aches and pains due to headache, muscular aches, backache. Common adverse reactions include pruritus, constipation, nausea, vomiting, headache, agitation, insomnia and atelectasis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- backache
- minor pain of arthritis
- the common cold
- toothache
- premenstrual and menstrual cramps
- temporarily reduces fever
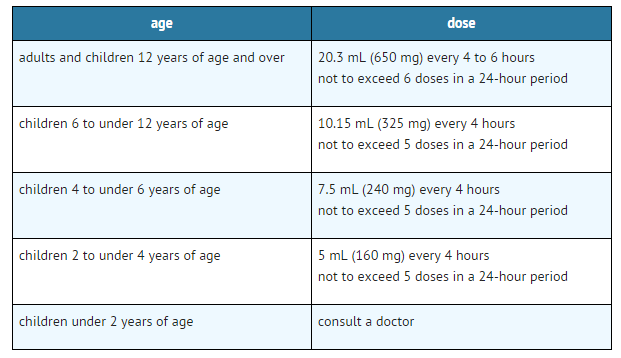
Dosing Information
- Do not take more than directed
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acetaminophen (rectal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetaminophen (rectal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Acetaminophen (rectal) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acetaminophen (rectal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetaminophen (rectal) in pediatric patients.
Contraindications
There is limited information regarding Acetaminophen (rectal) Contraindications in the drug label.
Warnings
- Liver warning
- This product contains acetaminophen. Severe liver damage may occur if:
- adult takes more than 6 doses in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours
- adult has 3 or more alcoholic drinks everyday while using this product
- Sore throat warning
- Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients of this product
- Ask a doctor before use if the user has liver disease.
- Ask a doctor or pharmacist before use if the user is taking the blood thinning drug warfarin.
- Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days in adults and children
- pain gets worse or lasts more than 5 days in children under 12 years
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
- These could be signs of a serious condition.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Acetaminophen (rectal) in the drug label.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Acetaminophen (rectal) in the drug label.
Drug Interactions
There is limited information regarding Acetaminophen (rectal) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- If pregnant or breast-feeding, ask a health professional before use.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acetaminophen (rectal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acetaminophen (rectal) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to nursing mothers.
Pediatric Use
- Keep out of reach of children.
Geriatic Use
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acetaminophen (rectal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acetaminophen (rectal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acetaminophen (rectal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acetaminophen (rectal) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Acetaminophen (rectal) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Acetaminophen (rectal) in the drug label.
Overdosage
- Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222). Quick medical attention is critical for adults as well as children even if you do not notice any signs or symptoms.
Pharmacology
Mechanism of Action
There is limited information regarding Acetaminophen (rectal) Mechanism of Action in the drug label.
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Acetaminophen (rectal) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Acetaminophen (rectal) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Acetaminophen (rectal) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Acetaminophen (rectal) in the drug label.
How Supplied
- sodium content: 2 mg/5 mLstore at 20° to 25°C (68° to 77°F). [See USP controlled room temperature]
- keep tightly closed
- protect from light
- a red, cherry flavored solution supplied in the following oral dosage forms:
- NDC 0121-0657-05 (unit dose cups of 5 mL, 10 × 10's), NDC 0121-0657-11 (unit dose cups of 10.15 mL, 10 × 10's), and NDC 0121-0657-21 (unit dose cups of 20.3 mL, 10 × 10's).
Storage
There is limited information regarding Acetaminophen (rectal) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Acetaminophen (rectal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Acetaminophen (rectal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Acetaminophen (rectal) in the drug label.
Precautions with Alcohol
- Alcohol-Acetaminophen (rectal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Acetaminophen (rectal) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Acetaminophen (rectal) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Template:Cite doi
- ↑ "melting point data for paracetamol". Lxsrv7.oru.edu. Retrieved 2011-03-19.
- ↑ Granberg RA, Rasmuson AC (1999). "Solubility of paracetamol in pure solvents". Journal of Chemical & Engineering Data. 44 (6): 1391–95. doi:10.1021/je990124v.
- ↑ Template:Cite isbn
- ↑ "Tylenol, Tylenol [[infants]]' Drops (acetaminophen) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 May 2014. URL–wikilink conflict (help)
- ↑ 6.0 6.1 6.2 "Codapane Forte Paracetamol and codeine phosphate PRODUCT INFORMATION" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 29 April 2013. Retrieved 10 May 2014.
{{#subobject:
|Label Page=Acetaminophen (rectal) |Label Name=Acetaminophen (rectal)03.png
}}
{{#subobject:
|Label Page=Acetaminophen (rectal) |Label Name=Acetaminophen (rectal)04.png
}}