Acetaminophen (rectal): Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag={{AV}} |genericName=acetaminophen(rectal) |aOrAn=an |drugClass=analgesic and antipyretic |indicationType=treatment |indication=m...") |
m (Protected "Acetaminophen (rectal)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (19 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=[[analgesic]] and [[antipyretic]] | |drugClass=[[analgesic]] and [[antipyretic]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=minor | |indication=[[fever]] and relieves minor [[aches]], [[pains]], and [[headache]] | ||
|adverseReactions=[[pruritus]], [[constipation]], [[nausea]], [[vomiting]], [[headache]], [[agitation]], [[insomnia]] and [[atelectasis]] | |adverseReactions=[[pruritus]], [[constipation]], [[nausea]], [[vomiting]], [[headache]], [[agitation]], [[insomnia]], and [[atelectasis]] | ||
|blackBoxWarningTitle=Title | |blackBoxWarningTitle=Title | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"> | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=There is limited information regarding <i>Adult Indications and Dosage</i> of {{PAGENAME}} in adult patients. | |||
|fdaLIADAdult= | |||
< | |||
< | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 41: | Line 18: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=*Temporarily | ||
:*reduces [[fever]] | |||
:*relieves minor aches, [[pains]], and [[headache]] | |||
====Direction to use==== | |||
*This product does not contain directions or complete warnings for adult use. | |||
:*Do not use more than directed | |||
:*Remove foil wrapper | |||
:*Insert suppository well up into rectum | |||
*Children 3-6 years | |||
:* 1 suppository every 4 to 6 hours while symptoms persist | |||
:* Do not exceed 5 suppositories in any 24-hour period | |||
*Children under 3 years: ask a doctor | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
| Line 52: | Line 39: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications=There is limited information regarding ''Contraindications'' of {{PAGENAME}} in the drug label. | ||
|warnings=* | |warnings=*For rectal use only | ||
*This product contains acetaminophen. Severe liver damage may occur if | *Liver Warning: This product contains acetaminophen. Severe [[liver damage]] may occur if your child inserts | ||
:* | :*more than 5 suppositories in 24 hours, which is the maximum daily amount | ||
:* | :*with other drugs containing acetaminophen | ||
: | *Allergy alert: Acetaminophen may cause severe skin reactions. | ||
*Symptoms may include: | |||
* | :*[[skin reddening]] | ||
:* | :*[[blisters]] | ||
:* [[rash]] | |||
*If a skin reaction occurs, stop use and seek medical help right away. | |||
*Do not use | *Do not use | ||
:*if your child is [[allergic]] to acetaminophen | |||
:*with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. | :*with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist. | ||
*Ask a doctor before use if your child has liver disease | |||
*Ask a doctor or pharmacist before use if your child is taking the blood thinning drug [[warfarin]] | |||
*Ask a doctor before use if | |||
*Ask a doctor or pharmacist before use if | |||
*Stop use and ask a doctor if | *Stop use and ask a doctor if | ||
:* | :*fever lasts more than 3 days (72 hours), or recurs | ||
:*pain | :*you need to use this product for pain for more than 5 days continuously | ||
*Severe or recurrent [[pain]], or high or continued fever may indicate a serious illness. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|clinicalTrials=There is limited information regarding <i>FDA-Labeled Indications and Dosage (Adult)</i> of {{PAGENAME}} in the drug label. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions=There is limited information regarding ''Drug Interactions'' of {{PAGENAME}} in the drug label. | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=* If pregnant or breast-feeding, ask a health professional before use. | |useInPregnancyFDA=* If pregnant or breast-feeding, ask a health professional before use. | ||
| Line 97: | Line 82: | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | ||
|useInPed=* | |useInPed=*If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for children even if you do not notice any signs or symptoms. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
| Line 107: | Line 92: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* Suppository | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 115: | Line 100: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=<!--Pharmacology--> | ||
<!--Pharmacology--> | |||
<!--Drug box 2--> | <!--Drug box 2--> | ||
| Line 131: | Line 114: | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = Tylenol (U.S. and Japan), Panadol (United Kingdom and Australia), and many others; see this thorough [[list of paracetamol brand names]] | | tradename = Tylenol (U.S. and Japan), Panadol (United Kingdom and Australia), and many others; see this thorough [[list of paracetamol brand names]] | ||
| MedlinePlus = a681004 | | MedlinePlus = a681004 | ||
| licence_US = Acetaminophen | | licence_US = Acetaminophen | ||
| Line 143: | Line 125: | ||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = 63-89% | | bioavailability = 63-89% | ||
| protein_bound = 10-25%<ref>{{cite web|title=Tylenol, Tylenol [[infants]]' Drops (acetaminophen) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=10 May 2014|url=http://reference.medscape.com/drug/tylenol-acetaminophen-343346#showall}}</ref> | | protein_bound = 10-25%<ref>{{cite web|title=Tylenol, Tylenol [[infants]]' Drops (acetaminophen) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|accessdate=10 May 2014|url=http://reference.medscape.com/drug/tylenol-acetaminophen-343346#showall}}</ref> | ||
| metabolism = Predominantly in the liver<ref name = TGA>{{cite web|title=Codapane Forte Paracetamol and codeine phosphate PRODUCT INFORMATION|work=TGA eBusiness Services|publisher=Alphapharm Pty Limited|date=29 April 2013|accessdate=10 May 2014|url=https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05623-3|format=PDF}}</ref> | | metabolism = Predominantly in the liver<ref name = TGA>{{cite web|title=Codapane Forte Paracetamol and codeine phosphate PRODUCT INFORMATION|work=TGA eBusiness Services|publisher=Alphapharm Pty Limited|date=29 April 2013|accessdate=10 May 2014|url=https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05623-3|format=PDF}}</ref> | ||
| Line 150: | Line 132: | ||
<!--Identifiers--> | <!--Identifiers--> | ||
| CASNo_Ref = | | CASNo_Ref = | ||
| CAS_number_Ref = | | CAS_number_Ref = | ||
| CAS_number = 103-90-2 | | CAS_number = 103-90-2 | ||
| ATC_prefix = N02 | | ATC_prefix = N02 | ||
| ATC_suffix = BE01 | | ATC_suffix = BE01 | ||
| PubChem = 1983 | | PubChem = 1983 | ||
| DrugBank_Ref = | | DrugBank_Ref = | ||
| DrugBank = DB00316 | | DrugBank = DB00316 | ||
| ChemSpiderID_Ref = | | ChemSpiderID_Ref = | ||
| ChemSpiderID = 1906 | | ChemSpiderID = 1906 | ||
| UNII_Ref = | | UNII_Ref = | ||
| UNII = 362O9ITL9D | | UNII = 362O9ITL9D | ||
| KEGG_Ref = | | KEGG_Ref = | ||
| KEGG = D00217 | | KEGG = D00217 | ||
| ChEBI_Ref = | | ChEBI_Ref = | ||
| ChEBI = 46195 | | ChEBI = 46195 | ||
| ChEMBL_Ref = | | ChEMBL_Ref = | ||
| ChEMBL = 112 | | ChEMBL = 112 | ||
| PDB_ligand = TYL | | PDB_ligand = TYL | ||
| Line 175: | Line 157: | ||
| smiles = CC(NC1=CC=C(O)C=C1)=O | | smiles = CC(NC1=CC=C(O)C=C1)=O | ||
| InChI = 1/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | | InChI = 1/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | ||
| StdInChI_Ref = | | StdInChI_Ref = | ||
| StdInChI = 1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | | StdInChI = 1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10) | ||
| StdInChIKey_Ref = | | StdInChIKey_Ref = | ||
| StdInChIKey = RZVAJINKPMORJF-UHFFFAOYSA-N | | StdInChIKey = RZVAJINKPMORJF-UHFFFAOYSA-N | ||
| density = 1.263 | | density = 1.263 | ||
| melting_point = 169 | | melting_point = 169 | ||
| melting_notes = | | melting_notes = | ||
}} | }} | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction= | |mechAction=There is limited information regarding ''Mechanism of Action'' of Acetaminophen (rectal) in the drug label. | ||
|structure=[[File:Acetaminophen structural formula.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |structure=[[File:Acetaminophen structural formula.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 201: | Line 182: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* For your safety, suppositories are packaged in tamper-evident sealed foil. Do not use if foil is torn or open. | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|storage=* Store at 8˚-25˚C (46˚-77˚F) | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames= | |brandNames=* ACEPHEN<ref>{{Cite web | title = ACEPHEN - acetaminophen suppository | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=32bbe845-7f0e-c33d-adb2-659f18fedfa9 }}</ref> | ||
|lookAlike=There is limited information regarding Look-Alike Drug Names. | |||
< | |||
|lookAlike= | |||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
| Line 228: | Line 201: | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}01.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}001.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
Latest revision as of 17:07, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acetaminophen (rectal) is an analgesic and antipyretic that is FDA approved for the treatment of fever and relieves minor aches, pains, and headache. Common adverse reactions include pruritus, constipation, nausea, vomiting, headache, agitation, insomnia, and atelectasis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Adult Indications and Dosage of Acetaminophen (rectal) in adult patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acetaminophen (rectal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetaminophen (rectal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Temporarily
Direction to use
- This product does not contain directions or complete warnings for adult use.
- Do not use more than directed
- Remove foil wrapper
- Insert suppository well up into rectum
- Children 3-6 years
- 1 suppository every 4 to 6 hours while symptoms persist
- Do not exceed 5 suppositories in any 24-hour period
- Children under 3 years: ask a doctor
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acetaminophen (rectal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetaminophen (rectal) in pediatric patients.
Contraindications
There is limited information regarding Contraindications of Acetaminophen (rectal) in the drug label.
Warnings
- For rectal use only
- Liver Warning: This product contains acetaminophen. Severe liver damage may occur if your child inserts
- more than 5 suppositories in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- Allergy alert: Acetaminophen may cause severe skin reactions.
- Symptoms may include:
- If a skin reaction occurs, stop use and seek medical help right away.
- Do not use
- if your child is allergic to acetaminophen
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- Ask a doctor before use if your child has liver disease
- Ask a doctor or pharmacist before use if your child is taking the blood thinning drug warfarin
- Stop use and ask a doctor if
- fever lasts more than 3 days (72 hours), or recurs
- you need to use this product for pain for more than 5 days continuously
- Severe or recurrent pain, or high or continued fever may indicate a serious illness.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding FDA-Labeled Indications and Dosage (Adult) of Acetaminophen (rectal) in the drug label.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Acetaminophen (rectal) in the drug label.
Drug Interactions
There is limited information regarding Drug Interactions of Acetaminophen (rectal) in the drug label.
Use in Specific Populations
Pregnancy
- If pregnant or breast-feeding, ask a health professional before use.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acetaminophen (rectal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acetaminophen (rectal) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to nursing mothers.
Pediatric Use
- If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for children even if you do not notice any signs or symptoms.
Geriatic Use
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acetaminophen (rectal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acetaminophen (rectal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acetaminophen (rectal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acetaminophen (rectal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acetaminophen (rectal) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Suppository
Monitoring
There is limited information regarding Monitoring of Acetaminophen (rectal) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Acetaminophen (rectal) in the drug label.
Overdosage
There is limited information regarding Acetaminophen (rectal) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Mechanism of Action of Acetaminophen (rectal) in the drug label.
Structure
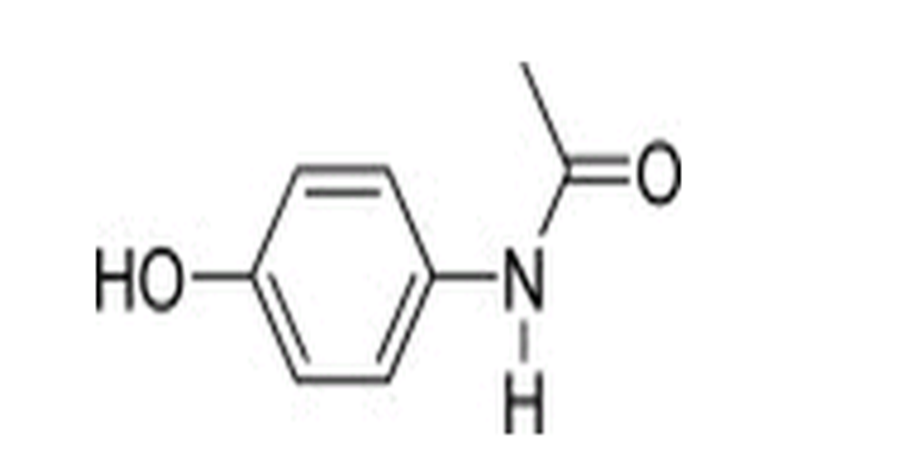
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Acetaminophen (rectal) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Acetaminophen (rectal) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Acetaminophen (rectal) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Acetaminophen (rectal) in the drug label.
How Supplied
- For your safety, suppositories are packaged in tamper-evident sealed foil. Do not use if foil is torn or open.
Storage
- Store at 8˚-25˚C (46˚-77˚F)
Images
Drug Images
{{#ask: Page Name::Acetaminophen (rectal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Acetaminophen (rectal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Acetaminophen (rectal) in the drug label.
Precautions with Alcohol
- Alcohol-Acetaminophen (rectal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ACEPHEN[3]
Look-Alike Drug Names
There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Tylenol, Tylenol [[infants]]' Drops (acetaminophen) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 10 May 2014. URL–wikilink conflict (help)
- ↑ 2.0 2.1 2.2 "Codapane Forte Paracetamol and codeine phosphate PRODUCT INFORMATION" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 29 April 2013. Retrieved 10 May 2014.
- ↑ "ACEPHEN - acetaminophen suppository".
{{#subobject:
|Label Page=Acetaminophen (rectal) |Label Name=Acetaminophen (rectal)01.png
}}
{{#subobject:
|Label Page=Acetaminophen (rectal) |Label Name=Acetaminophen (rectal)001.png
}}