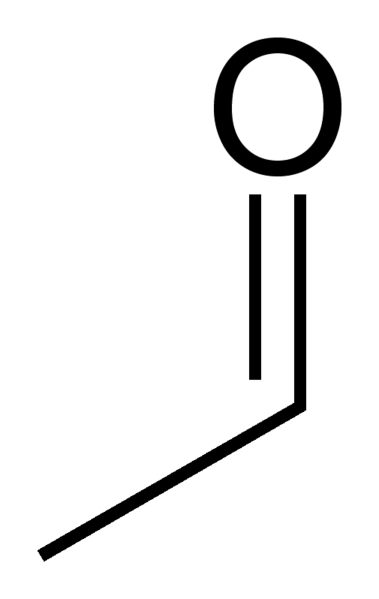
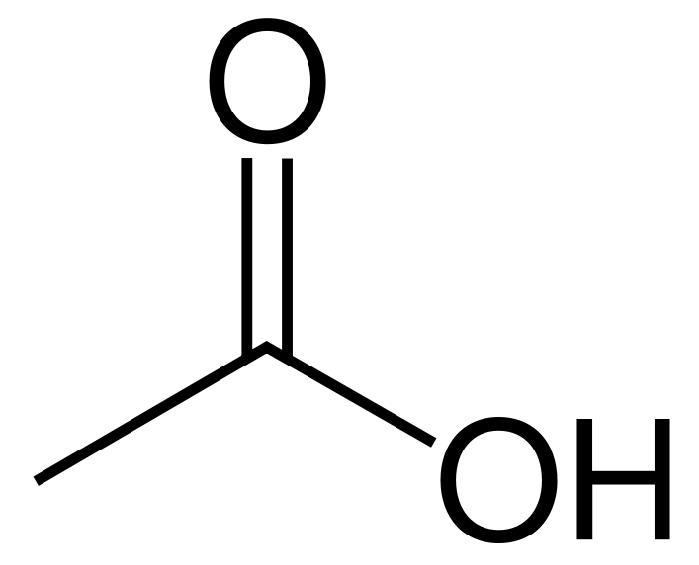
ALDH2
| Aldehyde dehydrogenase 2 family (mitochondrial) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1a4z. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | ALDH2 ; ALDH-E2; ALDHI; ALDM; MGC1806 | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 55480 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
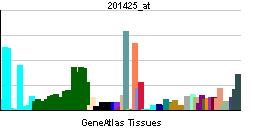 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Aldehyde dehydrogenase 2 family (mitochondrial), also known as ALDH2, is a human gene.
This protein belongs to the aldehyde dehydrogenase family of proteins. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Two major liver isoforms of this enzyme, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities, kinetic properties, and subcellular localizations. Most Caucasians have two major isozymes, while approximately 50% of Orientals have only the cytosolic isozyme, missing the mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among Orientals than among Caucasians could be related to the absence of the mitochondrial isozyme. This gene encodes a mitochondrial isoform, which has a low Km for acetaldehydes, and is localized in mitochondrial matrix.[1]
The enzyme associated with the chemical transformation from acetaldehyde to acetic acid is aldehyde dehydrogenase 2 family (ALDH2). The gene encoding for this enzyme is 1.2.1.3 and is found on chromosome 12.
See also
References
Further reading
- Yoshida A (1993). "Molecular genetics of human aldehyde dehydrogenase". Pharmacogenetics. 2 (4): 139–47. PMID 1306115.
- Chao YC, Liou SR, Tsai SF, Yin SJ (1994). "Dominance of the mutant ALDH2(2) allele in the expression of human stomach aldehyde dehydrogenase-2 activity". Proc. Natl. Sci. Counc. Repub. China B. 17 (3): 98–102. PMID 8290656.
- Seitz HK, Meier P (2007). "The role of acetaldehyde in upper digestive tract cancer in alcoholics". Translational research : the journal of laboratory and clinical medicine. 149 (6): 293–7. doi:10.1016/j.trsl.2006.12.002. PMID 17543846.
- Crabb DW, Edenberg HJ, Bosron WF, Li TK (1989). "Genotypes for aldehyde dehydrogenase deficiency and alcohol sensitivity. The inactive ALDH2(2) allele is dominant". J. Clin. Invest. 83 (1): 314–6. PMID 2562960.
- Hsu LC, Bendel RE, Yoshida A (1988). "Genomic structure of the human mitochondrial aldehyde dehydrogenase gene". Genomics. 2 (1): 57–65. PMID 2838413.
- Hsu LC, Tani K, Fujiyoshi T; et al. (1985). "Cloning of cDNAs for human aldehyde dehydrogenases 1 and 2". Proc. Natl. Acad. Sci. U.S.A. 82 (11): 3771–5. PMID 2987944.
- Braun T, Grzeschik KH, Bober E; et al. (1986). "The structural gene for the mitochondrial aldehyde dehydrogenase maps to human chromosome 12". Hum. Genet. 73 (4): 365–7. PMID 3017845.
- Braun T, Bober E, Singh S; et al. (1987). "Isolation and sequence analysis of a full length cDNA clone coding for human mitochondrial aldehyde dehydrogenase". Nucleic Acids Res. 15 (7): 3179. PMID 3562250.
- Braun T, Bober E, Singh S; et al. (1987). "Evidence for a signal peptide at the amino-terminal end of human mitochondrial aldehyde dehydrogenase". FEBS Lett. 215 (2): 233–6. PMID 3582651.
- Agarwal DP, Goedde HW (1987). "Human aldehyde dehydrogenase isozymes and alcohol sensitivity". Isozymes Curr. Top. Biol. Med. Res. 16: 21–48. PMID 3610592.
- Hempel J, Höög JO, Jörnvall H (1987). "Mitochondrial aldehyde dehydrogenase. Homology of putative targeting sequence to that of carbamyl phosphate synthetase I revealed by correlation of cDNA and protein data". FEBS Lett. 222 (1): 95–8. PMID 3653404.
- Yoshida A, Ikawa M, Hsu LC, Tani K (1985). "Molecular abnormality and cDNA cloning of human aldehyde dehydrogenases". Alcohol. 2 (1): 103–6. PMID 4015823.
- Hempel J, Kaiser R, Jörnvall H (1985). "Mitochondrial aldehyde dehydrogenase from human liver. Primary structure, differences in relation to the cytosolic enzyme, and functional correlations". Eur. J. Biochem. 153 (1): 13–28. PMID 4065146.
- Yoshida A, Huang IY, Ikawa M (1984). "Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals". Proc. Natl. Acad. Sci. U.S.A. 81 (1): 258–61. PMID 6582480.
- Xiao Q, Weiner H, Johnston T, Crabb DW (1995). "The aldehyde dehydrogenase ALDH2*2 allele exhibits dominance over ALDH2*1 in transduced HeLa cells". J. Clin. Invest. 96 (5): 2180–6. PMID 7593603.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. PMID 8125298.
- Novoradovsky A, Tsai SJ, Goldfarb L; et al. (1996). "Mitochondrial aldehyde dehydrogenase polymorphism in Asian and American Indian populations: detection of new ALDH2 alleles". Alcohol. Clin. Exp. Res. 19 (5): 1105–10. PMID 8561277.
- Xiao Q, Weiner H, Crabb DW (1997). "The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced flushing increases turnover of the enzyme tetramers in a dominant fashion". J. Clin. Invest. 98 (9): 2027–32. PMID 8903321.
External links
- ALDH2+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
| Stub icon | This biochemistry article is a stub. You can help Wikipedia by expanding it. |