Digoxin immune fab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Digoxin immune fab is a Immunoglobulin G antidote that is FDA approved for the {{{indicationType}}} of potentially life-threatening digoxin toxicity or overdose. Common adverse reactions include hypokalemia, exacerbation of low cardiac output, and rapid ventricular response in patients with atrial fibrillation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Digoxin immune fab in adult patients.
Non–Guideline-Supported Use
Toxic Ingestions of Lanatoside C
- Dosing Information
- Fab fragments (500 mg) were added to 500 mL dextrose 5% in water and infused continuously at 32 drops per minute over a total period of 5.5 hours (total dose, 460 mg).[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Digoxin immune fab in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Digoxin immune fab in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Digoxin immune fab in pediatric patients.
Contraindications
- There are no known contraindications to the use of DigiFab.
Warnings
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Digoxin immune fab in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Digoxin immune fab in the drug label.
Central Nervous System
Cardiovascular
Respiratory
Gastrointestinal
Hypersensitivity
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Digoxin immune fab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Digoxin immune fab during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Digoxin immune fab with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Digoxin immune fab with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Digoxin immune fab with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Digoxin immune fab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Digoxin immune fab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Digoxin immune fab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Digoxin immune fab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Digoxin immune fab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Digoxin immune fab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
- All patients should be informed of the possibility of an anaphylactic reaction and when receiving DigiFab should be carefully monitored for signs and symptoms of an acute allergic reaction (e.g., urticaria, pruritus, erythema, angioedema, bronchospasm with wheezing or cough, stridor, laryngeal edema, hypotension, tachycardia) and treated immediately with appropriate emergency medical care (e.g., oxygen, diphenhydramine, corticosteroids, volume expansion and airway management).
- Patients should be closely monitored, including temperature, blood pressure, electrocardiogram, and potassium concentration, during and after administration of DigiFab.
- Patients with severe renal failure who receive DigiFab for digitalis toxicity should be monitored for a prolonged period for possible recurrence of toxicity. Monitoring of free (unbound) digoxin concentrations after the administration may be appropriate in order to establish recrudescent toxicity in renal failure patients.
IV Compatibility
There is limited information regarding IV Compatibility of Digoxin immune fab in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Digoxin immune fab in the drug label.
Pharmacology
Digoxin immune fab
| |
| Systematic (IUPAC) name | |
| Anti-digoxin antibody fragment | |
| Identifiers | |
| CAS number | ? |
| ATC code | V03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 144602.257 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 15 hours for DigiFab, 23 hours for Digibind |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | IV infusion, injection |
Mechanism of Action
- DigiFab has an affinity for digoxin in the range of 109 to 1010 M-1, which is greater than the affinity of digoxin for its sodium pump receptor, the presumed receptor for its therapeutic and toxic effects. When administered to the intoxicated patient, DigiFab binds to molecules of digoxin reducing free digoxin levels, which results in a shift in the equilibrium away from binding to the receptors, thereby reducing cardio-toxic effects. Fab-digoxin complexes are then cleared by the kidney and reticuloendothelial system.
Structure
- DigiFab® [Digoxin Immune Fab (Ovine)] is a sterile, purified, lyophilized preparation of digoxin-immune ovine Fab (monovalent) immunoglobulin fragments. These fragments are obtained from the blood of healthy sheep immunized with a digoxin derivative, digoxin-dicarboxymethoxylamine (DDMA), a digoxin analogue which contains the functionally essential cyclopentaperhydrophenanthrene:lactone ring moiety coupled to keyhole limpet hemocyanin (KLH).
- The final product is prepared by isolating the immunoglobulin fraction of the ovine serum, digesting it with papain and isolating the digoxin-specific Fab fragments by affinity
chromatography. These antibody fragments have a molecular weight of approximately 46,000 Da.
- Each vial of DigiFab, which will bind approximately 0.5 mg digoxin, contains 40 mg of digoxin immune Fab, 75 mg (approx) of mannitol USP, and 2 mg (approx) sodium acetate USP as a buffering agent.
- The product contains no preservatives and is intended for intravenous administration after reconstitution with 4 mL of Sterile Water for Injection USP.
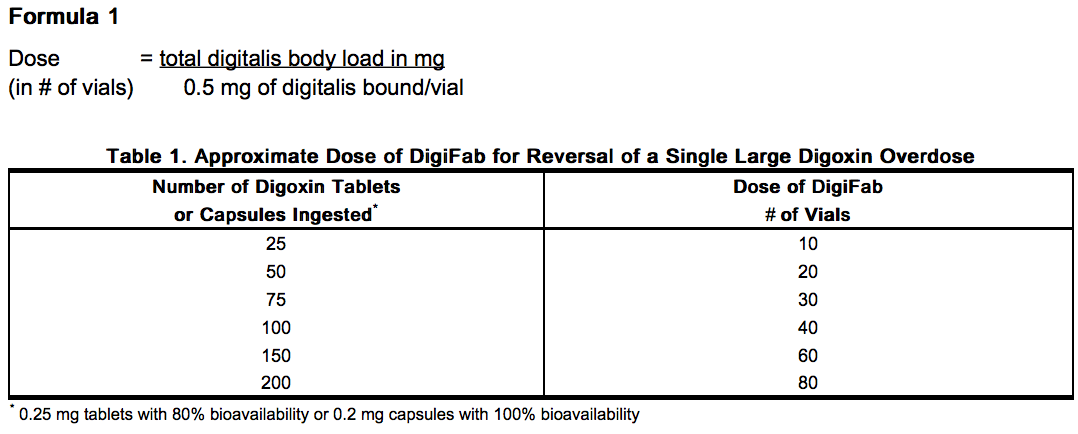
Pharmacodynamics
- No toxic effects were observed when DigiFab was administered to healthy male Sprague Dawley rats in equimolar doses sufficient to neutralize a 1 mg/kg dose of digoxin. In these studies, the physiologic changes produced by toxic serum concentrations of digoxin were ameliorated rapidly by the administration of DigiFab, or another ovine digoxin-specific immune Fab, Digibind® (manufactured by GlaxoSmithKline). Statistically equivalent responses were observed with both DigiFab and Digibind to the following variables: PTQ index, heart rate, mean arterial pressure, ventilation, arterial blood gases, and serum potassium concentrations.
Pharmacokinetics
- The pharmacokinetics of DigiFab were assessed in a randomized and controlled study of DigiFab and Digibind (comparator Fab product for treatment of digoxin toxicity). Sixteen healthy subjects were given 1 mg of intravenous digoxin followed by an approximately equimolar neutralizing dose of either DigiFab (n=8) or Digibind (n=8). The pharmacokinetic profiles of Fab were similar for both products.1 The similar volumes of distribution (0.3 L/kg and 0.4 L/kg for DigiFab and Digibind, respectively) indicate considerable penetration from the circulation into the extracellular space and are consistent with previous reports of ovine Fab distribution, as are the elimination half-life values (15 hours and 23 hours for DigiFab and Digibind, respectively).2-6 The elimination half-life of 15-20 hours in patients with normal renal function appears to be increased up to 10 fold in patients with renal impairment, although volume of distribution remains unaffected.6
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Digoxin immune fab in the drug label.
Clinical Studies
- There have been two controlled clinical trials conducted with DigiFab: a pharmacokinetic and pharmacodynamic study of DigiFab as compared to Digibind in healthy volunteers, and a prospective multi-center study of the efficacy of DigiFab in patients presenting with life-threatening digoxin toxicity.
- The objective of the pharmacokinetic and pharmacodynamic study was to compare these parameters for DigiFab to those for Digibind.1 This trial was conducted in healthy volunteers who were administered a 1 mg intravenous dose of digoxin, followed 2 hours later by an equimolar neutralizing dose of either DigiFab or Digibind. The pharmacokinetics of both digoxin and Fab were determined (see Clinical Pharmacokinetics for Fab pharmacokinetic parameters). The primary outcome measure was the serum level of free (unbound) digoxin. The results demonstrated that both products reduced the level of free digoxin in the serum to below the limit of assay quantitation for several hours after Fab administration. Cumulative urinary excretion of digoxin was comparable for both products and exceeded 40% of the administered dose by 24 hours. These results demonstrate that DigiFab and Digibind have equivalent pharmacodynamic effects on the digoxin parameters that are relevant to the treatment of digoxin toxicity. In this study, no patients developed a measurable immune response (human anti-ovine antibodies) to DigiFab.
- The objective of the efficacy study was to demonstrate safety and also to determine the pharmacokinetics of, and clinical response to, DigiFab in patients. Results were compared to historical data on another U.S. marketed ovine digoxin immune Fab product, Digibind. Fifteen patients received doses of DigiFab based on its theoretical binding capacity for digoxin, and based on the known amount of digoxin ingested or on blood concentrations of digoxin at the time of admission. This study was conducted in both the U.S. and in Finland.
- The primary outcome of the study was met in that serum free digoxin concentrations in all patients fell to undetectable levels following DigiFab administration. This was an expected outcome that is consistent with data in the literature showing that free digoxin concentrations fall rapidly following administration of Digibind.2 In the DigiFab trial, an independent blinded review of each patient's ECG showed that 10 of the 15 patients studied had ECG abnormalities that improved within 4 hours after the DigiFab infusion. The remaining 5 patients had ECG abnormalities that were unchanged from baseline throughout the 24-hour assessment period, and in one case through the 30-day follow up period. Although the reason for the lack of ECG resolution could not be clearly determined in all cases, it is possible that the ECG abnormalities observed in these patients were not entirely due to digoxin toxicity, but rather to another underlying cardiac problem. Assessing all manifestations of toxicity, investigators classified 7 out of the 15 patients (47%) studied as having complete resolution of digoxin toxicity within 4 hours of DigiFab administration, and 14 patients (93%) were classified as having resolved their digoxin toxicity by 20 hours. The data for the proportion of patients who responded to treatment with DigiFab is similar to, and consistent with, historical data available for Digibind.2-3 In this study, where 2/15 patients had serum available for human anti-ovine antibody determination, there was no measurable immune response.
How Supplied
Storage
There is limited information regarding Digoxin immune fab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Digoxin immune fab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Digoxin immune fab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Digoxin immune fab in the drug label.
Precautions with Alcohol
- Alcohol-Digoxin immune fab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Digibind®[2]
- Digifab®[3]
Look-Alike Drug Names
- A® — B®[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Hess, T. (1979-12). "Treatment of a case of lanatoside C intoxication with digoxin-specific F(ab')2 antibody fragments". American Heart Journal. 98 (6): 767–771. ISSN 0002-8703. PMID 495429. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "DIGIFAB (ovine digoxin immune fab) injection, powder, lyophilized, for solution".
- ↑ "DIGIFAB (ovine digoxin immune fab) injection, powder, lyophilized, for solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Digoxin immune fab |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Digoxin immune fab |Label Name=Digoxin immune fab07.png
}}
{{#subobject:
|Label Page=Digoxin immune fab |Label Name=Digoxin immune fab08.png
}}