Trandolapril description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2]
DESCRIPTION
Trandolapril is the ethyl ester prodrug of a nonsulfhydryl angiotensin converting enzyme (ACE) inhibitor, trandolaprilat. Trandolapril is chemically described as (2S, 3aR, 7aS)-1-[(S)-N-[(S)-1-Carboxy-3-phenylpropyl]alanyl] hexahydro-2-indolinecarboxylic acid, 1-ethyl ester. Its empirical formula is C24H34N2O5 and its structural formula is
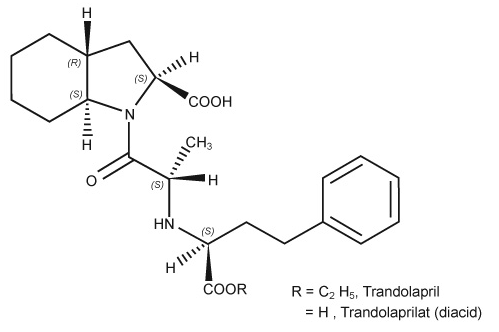 |
M.W. = 430.54
Melting Point = 125°C
Trandolapril is a white or almost white powder that is soluble (> 100 mg/mL) in chloroform, dichloromethane, and methanol. MAVIK tablets contain 1 mg, 2 mg, or 4 mg of trandolapril for oral administration. Each tablet also contains corn starch, croscarmellose sodium, hypromellose, iron oxide, lactose monohydrate, povidone, sodium stearyl fumarate.[1]
References
Adapted from the FDA Package Insert.