Alzheimer's disease medical therapy
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
Alzheimer's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Alzheimer's disease medical therapy On the Web |
|
American Roentgen Ray Society Images of Alzheimer's disease medical therapy |
|
Risk calculators and risk factors for Alzheimer's disease medical therapy |
Overview
There is no known cure for Alzheimer's disease. Available treatments offer relatively small symptomatic benefit but remain palliative in nature. Current treatments can be divided into pharmaceutical, psychosocial and caregiving.
Medical Therapy
Pharmacotherapies
Chronic Pharmacotherapies

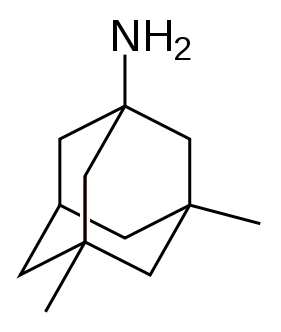
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) currently approve four medications to treat the cognitive manifestations of AD. Three are acetylcholinesterase inhibitors and the other is memantine, an NMDA receptor antagonist. No drug is currently able to delay or halt the progression of the disease.
Because reduction in cholinergic neuronal activity is well known in Alzheimer's disease,[1] acetylcholinesterase inhibitors are employed to reduce the rate at which acetylcholine (ACh) is broken down. This increases the concentration of ACh in the brain, thereby combatting the loss of ACh caused by the death of the cholinergic neurons.[2] Cholinesterase inhibitors currently approved include donepezil (brand name Aricept),[3] galantamine (Razadyne),[4] and rivastigmine (branded as Exelon,[5] and Exelon Patch[6]). There is also evidence for the efficacy of these medications in mild to moderate Alzheimer’s disease,[7] and some evidence for their use in the advanced stage. Only donepezil is approved for treatment of advanced AD dementia.[8] The use of these drugs in mild cognitive impairment has not shown any effect in delaying the onset of AD.[9] The most common side effects include nausea and vomiting, both of which are linked to cholinergic excess. These side effects arise in approximately ten to twenty percent of users and are mild to moderate in severity. Less common secondary effects include muscle cramps; decreased heart rate (bradycardia), decreased appetite and weight, and increased gastric acid.[10][11][12][13]
Glutamate is an excitatory neurotransmitter of the nervous system. Excessive amounts of glutamate in the brain can lead to cell death through a process called excitotoxicity which consists of the overstimulation of glutamate receptors. Excitotoxicity occurs not only in Alzheimer's disease, but also in other neurological diseases such as Parkinson's disease and multiple sclerosis.[14] Memantine (brand names Akatinol, Axura, Ebixa/Abixa, Memox and Namenda),[15] is a noncompetitive NMDA receptor antagonist first used as an anti-influenza agent. It acts on the glutamatergic system by blocking NMDA glutamate receptors and inhibits their overstimulation by glutamate.[14] Memantine has been shown to be moderately efficacious in the treatment of moderate to severe Alzheimer’s disease. Its effects in the initial stages of AD are unknown.[16] Reported adverse events with memantine are infrequent and mild, including hallucinations, confusion, dizziness, headache and fatigue.[17] Memantine used in combination with donepezil has been shown to be "of statistically significant but clinically marginal effectiveness".[18]
Neuroleptic anti-psychotic drugs commonly given to Alzheimer's patients with behavioural problems are modestly useful in reducing aggression and psychosis, but are associated with serious adverse effects, such as cerebrovascular events, movement difficulties or cognitive decline. These side effects do not permit the routine use of these medications.[19][20][21]
References
- ↑ Geula C, Mesulam MM (1995). "Cholinesterases and the pathology of Alzheimer disease". Alzheimer Dis Assoc Disord. 9 Suppl 2: 23–8. PMID 8534419.
- ↑ Stahl SM (2000). "The new cholinesterase inhibitors for Alzheimer's disease, Part 2: illustrating their mechanisms of action". J Clin Psychiatry. 61 (11): 813–814. PMID 11105732.
- ↑ "Donepezil". US National Library of Medicine (Medline). 2007-01-08. Retrieved 2008-03-20.
- ↑ "Galantamine". US National Library of Medicine (Medline). 2007-01-08. Retrieved 2008-03-20.
- ↑ "Rivastigmine". US National Library of Medicine (Medline). 2007-01-08. Retrieved 2008-03-20.
- ↑ "Rivastigmine Transdermal". US National Library of Medicine (Medline). 2007-01-08. Retrieved 2008-03-20.
- ↑ Birks J (2006). "Cholinesterase inhibitors for Alzheimer's disease". Cochrane Database Syst Rev (1): CD005593. doi:10.1002/14651858.CD005593. PMID 16437532.
- ↑ Birks J, Harvey RJ (2006). "Donepezil for dementia due to Alzheimer's disease". Cochrane Database Syst Rev (1): CD001190. doi:10.1002/14651858.CD001190.pub2. PMID 16437430.
- ↑ Raschetti R, Albanese E, Vanacore N, Maggini M (2007). "Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials". PLoS Med. 4 (11): e338. doi:10.1371/journal.pmed.0040338. PMID 18044984.
- ↑ "Aricept and Aricept ODT Product Insert" (PDF). Eisai and Pfizer. Retrieved 2008-01-30.
- ↑ "Razadyne ER U.S. Full Prescribing Information" (PDF). Ortho-McNeil Neurologics. Retrieved 2008-02-19.
- ↑ "Exelon ER U.S. Prescribing Information" (PDF). Novartis Pharmaceuticals. Retrieved 2008-02-19.
- ↑ "Exelon U.S. Prescribing Information" (PDF). Novartis Pharmaceuticals. Retrieved 2008-02-21.
- ↑ 14.0 14.1 Lipton SA (2006). "Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond". Nat Rev Drug Discov. 5 (2): 160–170. doi:10.1038/nrd1958. PMID 16424917.
- ↑ "Memantine". US National Library of Medicine (Medline). 2004-01-04. Retrieved 2008-03-22.
- ↑ Areosa Sastre A, McShane R, Sherriff F (2004). "Memantine for dementia". Cochrane Database Syst Rev (4): CD003154. doi:10.1002/14651858.CD003154.pub2. PMID 15495043.
- ↑ "Namenda Prescribing Information" (PDF). Forest Pharmaceuticals. Retrieved 2008-02-19.
- ↑ Raina P, Santaguida P, Ismaila A; et al. (2008). "Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline". Annals of Internal Medicine. 148 (5): 379–397. PMID 18316756.
- ↑ Ballard C, Waite J (2006). "The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease". Cochrane Database Syst Rev (1): CD003476. doi:10.1002/14651858.CD003476.pub2. PMID 16437455.
- ↑ Ballard C, Lana MM, Theodoulou M; et al. (2008). "A Randomised, Blinded, Placebo-Controlled Trial in Dementia Patients Continuing or Stopping Neuroleptics (The DART-AD Trial)". PLoS Med. 5 (4): e76. doi:10.1371/journal.pmed.0050076. PMID 18384230.
- ↑ Sink KM, Holden KF, Yaffe K (2005). "Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence". JAMA. 293 (5): 596–608. doi:10.1001/jama.293.5.596. PMID 15687315.