Malaria
For patient information click here
|
Malaria Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Malaria On the Web |
|
American Roentgen Ray Society Images of Malaria |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
| Malaria | ||
 | ||
|---|---|---|
| Plasmodium falciparum ring-forms and gametocytes in human blood. | ||
| ICD-10 | B50 | |
| ICD-9 | 084 | |
| DiseasesDB | 7728 | |
| MedlinePlus | 000621 | |
| eMedicine | med/1385 emerg/305 ped/1357 | |
| MeSH | C03.752.250.552 | |
Overview
Historical perspective
Epidemiology & Demographics
History & Symptoms
Symptoms of malaria include fever, shivering, arthralgia (joint pain), vomiting, anemia caused by hemolysis, hemoglobinuria, and convulsions. There may be the feeling of tingling in the skin, particularly with malaria caused by P. falciparum. The classical symptom of malaria is cyclical occurrence of sudden coldness followed by rigor and then fever and sweating lasting four to six hours, occurring every two days in P. vivax and P. ovale infections, while every three for P. malariae.[1] P. falciparum can have recurrent fever every 36-48 hours or a less pronounced and almost continuous fever. For reasons that are poorly understood, but which may be related to high intracranial pressure, children with malaria frequently exhibit abnormal posturing, a sign indicating severe brain damage.[2] Malaria has been found to cause cognitive impairments, especially in children. It causes widespread anemia during a period of rapid brain development and also direct brain damage. This neurologic damage results from cerebral malaria to which children are more vulnerable.[3]
Severe malaria is almost exclusively caused by P. falciparum infection and usually arises 6-14 days after infection.[4] Consequences of severe malaria include coma and death if untreated—young children and pregnant women are especially vulnerable. Splenomegaly (enlarged spleen), severe headache, cerebral ischemia, hepatomegaly (enlarged liver), hypoglycemia, and hemoglobinuria with renal failure may occur. Renal failure may cause blackwater fever, where hemoglobin from lysed red blood cells leaks into the urine. Severe malaria can progress extremely rapidly and cause death within hours or days.[4] In the most severe cases of the disease fatality rates can exceed 20%, even with intensive care and treatment.[5] In endemic areas, treatment is often less satisfactory and the overall fatality rate for all cases of malaria can be as high as one in ten.[6] Over the longer term, developmental impairments have been documented in children who have suffered episodes of severe malaria.[7]
Chronic malaria is seen in both P. vivax and P. ovale, but not in P. falciparum. Here, the disease can relapse months or years after exposure, due to the presence of latent parasites in the liver. Describing a case of malaria as cured by observing the disappearance of parasites from the bloodstream can therefore be deceptive. The longest incubation period reported for a P. vivax infection is 30 years.[4] Approximately one in five of P. vivax malaria cases in temperate areas involve overwintering by hypnozoites (i.e., relapses begin the year after the mosquito bite).[8]
Causes of Malaria
Pathophysiology
Evolutionary pressure of malaria on human genes
Malaria is thought to have been the greatest selective pressure on the human genome in recent history.[9] This is due to the high levels of mortality and morbidity caused by malaria, especially the P. falciparum species.
Sickle-cell disease
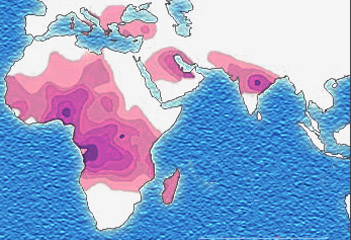
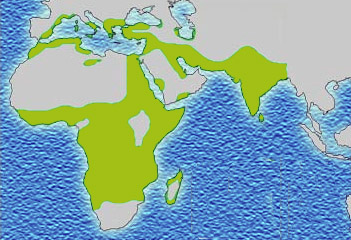
The best-studied influence of the malaria parasite upon the human genome is the blood disease, sickle-cell disease. In sickle-cell disease, there is a mutation in the HBB gene, which encodes the beta globin subunit of haemoglobin. The normal allele encodes a glutamate at position six of the beta globin protein, while the sickle-cell allele encodes a valine. This change from a hydrophilic to a hydrophobic amino acid encourages binding between haemoglobin molecules, with polymerization of haemoglobin deforming red blood cells into a "sickle" shape. Such deformed cells are cleared rapidly from the blood, mainly in the spleen, for destruction and recycling.
In the merozoite stage of its life cycle the malaria parasite lives inside red blood cells, and its metabolism changes the internal chemistry of the red blood cell. Infected cells normally survive until the parasite reproduces, but if the red cell contains a mixture of sickle and normal haemoglobin, it is likely to become deformed and be destroyed before the daughter parasites emerge. Thus, individuals heterozygous for the mutated allele, known as sickle-cell trait, may have a low and usually unimportant level of anaemia, but also have a greatly reduced chance of serious malaria infection. This is a classic example of heterozygote advantage.
Individuals homozygous for the mutation have full sickle-cell disease and in traditional societies rarely live beyond adolescence. However, in populations where malaria is endemic, the frequency of sickle-cell genes is around 10%. The existence of four haplotypes of sickle-type hemoglobin suggests that this mutation has emerged independently at least four times in malaria-endemic areas, further demonstrating its evolutionary advantage in such affected regions. There are also other mutations of the HBB gene that produce haemoglobin molecules capable of conferring similar resistance to malaria infection. These mutations produce haemoglobin types HbE and HbC which are common in Southeast Asia and Western Africa, respectively.
Thalassaemias
Another well documented set of mutations found in the human genome associated with malaria are those involved in causing blood disorders known as thalassaemias. Studies in Sardinia and Papua New Guinea have found that the gene frequency of β-thalassaemias is related to the level of malarial endemicity in a given population. A study on more than 500 children in Liberia found that those with β-thalassaemia had a 50% decreased chance of getting clinical malaria. Similar studies have found links between gene frequency and malaria endemicity in the α+ form of α-thalassaemia. Presumably these genes have also been selected in the course of human evolution.
Duffy antigens
The Duffy antigens are antigens expressed on red blood cells and other cells in the body acting as a chemokine receptor. The expression of Duffy antigens on blood cells is encoded by Fy genes (Fya, Fyb, Fyc etc.). Plasmodium vivax malaria uses the Duffy antigen to enter blood cells. However, it is possible to express no Duffy antigen on red blood cells (Fy-/Fy-). This genotype confers complete resistance to P. vivax infection. The genotype is very rare in European, Asian and American populations, but is found in almost all of the indigenous population of West and Central Africa.[10] This is thought to be due to very high exposure to P. vivax in Africa in the last few thousand years.
G6PD
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme which normally protects from the effects of oxidative stress in red blood cells. However, a genetic deficiency in this enzyme results in increased protection against severe malaria.
HLA and interleukin-4
HLA-B53 is associated with low risk of severe malaria. This MHC class I molecule presents liver stage and sporozoite antigens to T-Cells. Interleukin-4, encoded by IL4, is produced by activated T cells and promotes proliferation and differentiation of antibody-producing B cells. A study of the Fulani of Burkina Faso, who have both fewer malaria attacks and higher levels of antimalarial antibodies than do neighboring ethnic groups, found that the IL4-524 T allele was associated with elevated antibody levels against malaria antigens, which raises the possibility that this might be a factor in increased resistance to malaria.[11]
Diagnosis
Lab Tests
Treatment
Medical Therapy
Primary Prevention
References
- ↑ Malaria life cycle & pathogenesis. Malaria in Armenia. Accessed October 31, 2006.
- ↑ Idro, R. "Decorticate, decerebrate and opisthotonic posturing and seizures in Kenyan children with cerebral malaria". Malaria Journal. 4 (57). PMID 16336645. Retrieved 2007-01-21. Unknown parameter
|coauthors=ignored (help) - ↑ Boivin, M.J., "Effects of early cerebral malaria on cognitive ability in Senegalese children," Journal of Developmental and Behavioral Pediatrics 23, no. 5 (October 2002): 353–64. Holding, P.A. and Snow, R.W., "Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence," American Journal of Tropical Medicine and Hygiene 64, suppl. nos. 1–2 (January–February 2001): 68–75.
- ↑ 4.0 4.1 4.2 Trampuz A, Jereb M, Muzlovic I, Prabhu R (2003). "Clinical review: Severe malaria". Crit Care. 7 (4): 315–23. PMID 12930555.
- ↑ Kain K, Harrington M, Tennyson S, Keystone J (1998). "Imported malaria: prospective analysis of problems in diagnosis and management". Clin Infect Dis. 27 (1): 142–9. PMID 9675468.
- ↑ Mockenhaupt F, Ehrhardt S, Burkhardt J, Bosomtwe S, Laryea S, Anemana S, Otchwemah R, Cramer J, Dietz E, Gellert S, Bienzle U (2004). "Manifestation and outcome of severe malaria in children in northern Ghana". Am J Trop Med Hyg. 71 (2): 167–72. PMID 15306705.
- ↑ Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung'ala-Odera V, Lees JA, Newton CR (2005). "Developmental impairments following severe falciparum malaria in children". Trop Med Int Health. 10: 3–10. PMID 15655008.
- ↑ Adak T, Sharma V, Orlov V (1998). "Studies on the Plasmodium vivax relapse pattern in Delhi, India". Am J Trop Med Hyg. 59 (1): 175–9. PMID 9684649.
- ↑ Kwiatkowski, DP (2005). "How Malaria Has Affected the Human Genome and What Human Genetics Can Teach Us about Malaria". Am J Hum Genet. 77: 171–92. PMID 16001361.
- ↑ Carter R, Mendis KN (2002). "Evolutionary and historical aspects of the burden of malaria". Clin. Microbiol. Rev. 15 (4): 564–94. PMID 12364370.
- ↑ Verra F, Luoni G, Calissano C, Troye-Blomberg M, Perlmann P, Perlmann H, Arcà B, Sirima B, Konaté A, Coluzzi M, Kwiatkowski D, Modiano D (2004). "IL4-589C/T polymorphism and IgE levels in severe malaria". Acta Trop. 90 (2): 205–9. PMID 15177147.
External links
- National Geographic July 2007 Issue on Malaria
- WHO site on malaria
- Template:McGrawHillAnimation
- Johns Hopkins Malariology Open Courseware
- www.malariacontrol.net distributed computing project for the fight against malaria
- United States Centers for Disease Control - Malaria information pages
- Doctors Without Borders/Medecins Sans Frontieres - Malaria information pages
- HRC/Eldis Health Resource Guide - Malaria research and resources on health in developing countries
- Medline Plus - Malaria
- Interview with Dr Andrew Speilman, Harvard malaria specialist
- Malaria Consortium website
- GlobalHealthFacts.org Malaria Cases and Deaths by Country
- Survey article: History of malaria around the North Sea
- DriveAgainstMalaria.org, "World's longest journey to fight the biggest killer of children"
- Malaria on JHSPH OpenCourseWare
- Malaria Foundation International
- Malaria Atlas Project
- UNITAID, International Facility for the Purchase of Drugs (Wikipedia Article)
- BBC - Hopes of Malaria Vaccine by 2010 15 October 2004
- BBC - Science shows how malaria hides 8 April 2005
- History of discoveries in malaria
- Malaria. The UNICEF-UNDP-World Bank-WHO Special Programme for Research and Training in Tropical Diseases
- Malaria Vaccine Initiative
- Story of the discovery of the vector of the malarial parasite
- Wellcome Trust against Malaria
- "Vaccines for Development" - Blog on vaccine research and production for developing countries
- Medicines for Malaria Venture
- Malaria and Mosquitos - questions and answers
- Hisnets - Fighting Malaria: One Net At A Time
- Call for Increased Production of Long-Lasting Insecticidal Nets as Part of the U.N. Millenium Campaign
- Providing everyone with a LLIN in Sahn Malen, a small village in Sierra Leone
- Burden of Malaria, BBC pictures relating to malaria in northern Uganda
- Malaria: Cooperation among Parasite, Vector, and Host (Animation)
Template:Link FA Template:Protozoal diseases
Template:Link FA Template:Link FA Template:Link FA af:Malaria ar:ملاريا zh-min-nan:Ma-lá-lí-á bs:Malarija bg:Малария ca:Malària cs:Malárie da:Malaria de:Malaria el:Ελονοσία eo:Malario eu:Malaria gl:Malaria ko:말라리아 hi:शीतज्वर hr:Malarija id:Malaria ia:Malaria it:Malaria he:מלריה ka:მალარია hu:Malária mt:Malarja ms:Malaria nl:Malaria no:Malaria om:Malaria ps:ملاريا qu:Chukchu simple:Malaria sk:Malária sl:Malarija sr:Маларија sh:Malarija su:Malaria fi:Malaria sv:Malaria ta:மலேரியா te:మలేరియా th:มาลาเรีย uk:Малярія