Dihydrodesoxymorphine
 | |
| Clinical data | |
|---|---|
| Synonyms | Desomorphine, Dihydrodesoxymorphine, Permonid |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
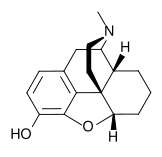
| Formula | C17H21NO2 |
| Molar mass | 271.354 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Desomorphine (Dihydrodesoxymorphine, Permonid) is an opiate analogue invented in 1933 in the United States, that is a derivative of morphine, where the 6-hydroxy group has been removed and the 7,8 double bond has been saturated. It has sedative and analgesic effects, and is around 10 times more potent than morphine.[1][2][3] It was used in Switzerland under the brand name Permonid, and was described as having a fast onset and a short duration of action, with relatively little nausea or respiratory depression compared to equivalent doses of morphine. This drug has attracted recent attention in Russia due to an upsurge in clandestine production, presumably due to its relatively simple synthesis from codeine.[4] It is prepared from α-chlorocodide, which is itself obtained by reacting thionyl chloride with codeine. By catalytic reduction, α-chlorocodide gives dihydrodesoxycodeine, which yields desomorphine on demethylation.[5][6]
References
- ↑ Bognar R, Makleit S. New method for the preparation of dihydro-6-desoxymorphine. (German). Arzneimittelforschung. 1958 Jun;8(6):323-5. PMID 13546093
- ↑ Janssen PA. A review of the chemical features associated with strong morphine-like activity. British Journal of Anaesthesia. 1962 Apr;34(4):260-268. PMID 14451235
- ↑ Sargent LJ, May EL. Agonists--antagonists derived from desomorphine and metopon. Journal of Medicinal Chemistry. 1970 Nov;13(6):1061-3. PMID 4098039
- ↑ Drug police RB is alarmed with growth of Desomorphine usage
- ↑ Mosettig E, Cohen FL, Small LF. Desoxycodeine Studies. III. The Constitution of the So-Called Alpha-Dehydrodesoxycodeine: Bis-Di-hydrodesoxycodeine. Journal of the American Chemical Society 1932; 54:793-801.
- ↑ Eddy NB, Howes HA. Studies of Morphine, Codeine and their Derivatives X. Desoxymorphine-C, Desoxycodeine-C and their Hydrogenated Derivatives. Journal of Pharmacology And Experimental Therapeutics. 1935; 55(3):257-267.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Opioids