Pegvaliase
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF ANAPHYLAXIS
See full prescribing information for complete Boxed Warning.
*Anaphylaxis has been reported after administration of Palynziq and may occur at any time during treatment.
|
Overview
Pegvaliase is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
RISK OF ANAPHYLAXIS
See full prescribing information for complete Boxed Warning.
*Anaphylaxis has been reported after administration of Palynziq and may occur at any time during treatment.
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pegvaliase in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Pegvaliase and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Pegvaliase
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Pegvaliase-pqpz is a PEGylated phenylalanine ammonia lyase (PAL) enzyme that converts phenylalanine to ammonia and trans‑cinnamic acid. It substitutes for the deficient phenylalanine hydroxylase (PAH) enzyme activity in patients with PKU and reduces blood phenylalanine concentrations.
Structure
(Description with picture)
Pharmacodynamics
- Palynziq treatment of adult patients with PKU resulted in the reduction of blood phenylalanine concentrations from pre-treatment baseline. The reduction of blood phenylalanine concentrations diminished with decreased pegvaliase‑pqpz plasma concentrations.
Pharmacokinetics
There is limited information regarding Pegvaliase Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity and genotoxicity studies have not been performed with pegvaliase‑pqpz. Based on its mechanism of action, pegvaliase‑pqpz is not expected to be tumorigenic.
- Pegvaliase-pqpz produced impaired fertility in female rats at 20 mg/kg/day subcutaneously (19.4 times the human steady‑state AUC at the maximum recommended daily dose), as indicated by decreases in corpora lutea, implantations, and litter size. These effects were associated with toxicity (decreased body weight, ovarian weight, and food consumption). No effects on mating or fertility were observed in female rats with 8 mg/kg/day subcutaneously (4.2 times the human steady‑state AUC at the maximum recommended daily dose) or in male rats with 20 mg/kg/day subcutaneously.
Animal Toxicology and/or Pharmacology
- In rats without PKU treated with pegvaliase-pqpz, dose-dependent vacuolation in multiple organs and tissues was observed in the 4‑ and 26‑week repeat dose toxicity studies at doses of 8 mg/kg subcutaneously or greater administered twice weekly (less than the human steady state AUC at the maximum recommended daily dose). Vacuolation occurred in renal tubule cells and in histiocytic cells of the liver, spleen, testes, adrenal cortex, mesenteric lymph node, and mandibular lymph node. Vacuolation in histiocytes of the affected organs and tissues persisted after cessation of treatment. The vacuolation observed in these studies was not associated with organ‑related toxicities as determined by clinical chemistry/urinalysis and histopathological examination. The clinical significance of these findings and functional consequences are unknown.
- In the 39‑week repeat dose toxicity study in monkeys, pegvaliase-pqpz 3 mg/kg subcutaneously twice weekly (3 times the human steady state AUC at the maximum recommended daily dose) produced systemic arteritis involving small arteries and arterioles in a wide range of organs and tissues (kidney, urinary bladder, pancreas, gallbladder, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, lung, heart, sciatic nerve, lacrimal gland, mandibular lymph node, epididymis, seminal vesicle, ovary, uterus, cervix, and vagina) and in subcutaneous injection sites. Arteritis was likely due to the immune-mediated response (e.g., immune complex deposition in blood vessels) associated with chronic administration of a foreign protein to the animals. The incidence and severity of systemic arteritis was dose-dependent. The vascular inflammation observed in this study was not associated with organ related toxicities as determined by clinical pathology parameters (hematology, clinical chemistry, and urinalysis) and histopathological examination.
- Studies of longer duration in rats and monkeys treated with pegvaliase‑pqpz have not been conducted.
Clinical Studies
Study 301: Induction/Titration/Maintenance Treatment
- Study 165‑301 (referred to as Study 301, NCT01819727) was an open-label randomized, multi-center study of adults with PKU to assess safety and tolerability of self-administered Palynziq in an induction/titration/maintenance regimen with a target maintenance dose of 20 mg subcutaneously once daily or 40 mg subcutaneously once daily. At Palynziq treatment initiation, 253 patients demonstrated inadequate blood phenylalanine control (blood phenylalanine concentration greater than 600 micromol/L) on existing management, and 8 patients had blood phenylalanine concentrations less than or equal to 600 micromol/L. Existing management options included prior or current restriction of dietary phenylalanine and protein intake, and/or prior treatment with sapropterin dihydrochloride. Patients previously treated with sapropterin dihydrochloride were required to discontinue use at least 14 days prior to the first dose.
- The 261 enrolled patients were aged 16 to 55 years (mean: 29 years) and had a baseline mean (range) blood phenylalanine of 1,233 (285, 2330) micromol/L. One hundred forty nine out of 261 (57%) patients were taking medical food at baseline and 41 out of 261 patients (16%) were on a protein-restricted diet at baseline (defined as receiving greater than 75% of total protein intake from medical food). Patients were randomized (1:1) to one of two target maintenance dosage arms: 20 mg once daily or 40 mg once daily. Patients were titrated to reach their randomized target dosage of 20 mg once daily or 40 mg once daily. The duration of titration varied among patients and was based on patient tolerability. Of the 261 enrolled patients, 195 (75%) patients reached their randomized maintenance dosage (103 in the 20 mg once daily arm, 92 in the 40 mg once daily arm). Among the patients who reached their randomized maintenance dosage, patients in the 20 mg once daily randomized arm reached their maintenance dosage at a median time of 10 weeks (range: 9 to 29 weeks) and patients in the 40 mg once daily arm reached their maintenance dosage at a median time of 11 weeks (range: 10 to 33 weeks).
- Of the 261 patients who enrolled in Study 301, 54 (21%) patients discontinued treatment during Study 301, 4 patients completed Study 301 and did not continue to Study 165‑302 (referred to as Study 302, NCT01889862), 152 patients continued to the eligibility period of Study 302, and 51 patients continued directly from Study 301 into the long‑term treatment period of Study 302.
Study 302: Efficacy Assessment
- A total of 164 adult patients with PKU who were previously-treated with Palynziq (152 patients from Study 301 and 12 patients from other Palynziq trials) enrolled in Study 302 and continued treatment with Palynziq in Study 302 for up to 13 weeks to assess eligibility for randomized withdrawal period.
Randomized Withdrawal Period
- Following this period of up to 13 weeks of additional Palynziq treatment in Study 302, eligibility for entry into the efficacy assessment period (randomized withdrawal period) was determined by whether a patient achieved at least a 20% reduction in blood phenylalanine concentration from pre-treatment baseline (when in previous studies). Eighty‑six out of 164 patients (52%) met this response target and continued into the randomized withdrawal period. In the double-blind, placebo-controlled, randomized withdrawal period, patients were randomized in a 2:1 ratio to either continue their maintenance Palynziq dosage or to receive matching placebo for a total of 8 weeks. The treatment difference in least squares (LS) mean change in blood phenylalanine concentration from the Study 302 randomized withdrawal baseline to randomized withdrawal Week 8 for each randomized study arm is shown in Table 5. Mean blood phenylalanine concentrations at pre-treatment baseline (Study 301 or other Palynziq trials) are also shown in Table 5. At Study 302 randomized withdrawal Week 8, Palynziq‑treated patients (20 mg once daily or 40 mg once daily) maintained their blood phenylalanine concentrations as compared to their randomized withdrawal baseline, whereas patients randomized to matching placebo (20 mg once daily or 40 mg once daily) returned to their pretreatment baseline blood phenylalanine concentrations (Figure 1).

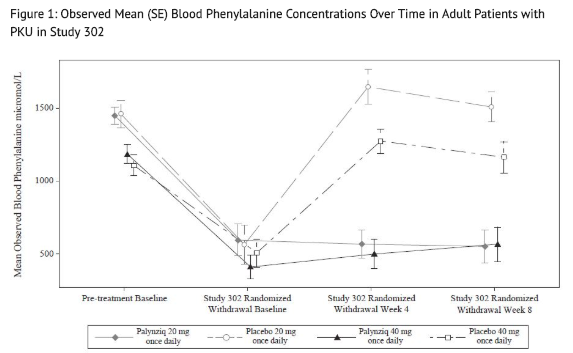
Study 301 and 302 Continuous Treatment
- Of 118 patients from Study 301 with a pre‑treatment baseline blood phenylalanine concentration greater than 600 micromol/L who were randomized to and received at least one dose of 20 mg once daily Palynziq, 108 patients, 98 patients, and 51 patients were treated for at least 24 weeks, 48 weeks, and 96 weeks, respectively.
- Of the 118 patients, 53 patients reached their first response (at least a 20% reduction in blood phenylalanine concentration from pre-treatment baseline or a blood phenylalanine concentration less than or equal to 600 micromol/L) by 4 weeks of treatment with 20 mg once daily and 28 patients reached their first response between Weeks 4 and 24 with 20 mg once daily. Of the 118 patients, 25 patients escalated their dosage from 20 mg once daily to 40 mg once daily before reaching a first response; of those 25 patients, 8 patients reached their first response by 4 weeks of treatment with 40 mg once daily and 6 patients reached their first response between Weeks 4 and 16 with 40 mg once daily.
How Supplied
- Palynziq (pegvaliase-pqpz) injection is supplied as a preservative-free, sterile, clear to slightly opalescent, colorless to pale yellow solution. All dosage strengths of Palynziq are provided in a 1 mL glass syringe with a 26 gauge, 0.5 inch needle.
- Each carton contains 1 or 10 trays with single-dose prefilled syringe(s), Prescribing Information, Medication Guide, and Instructions for Use. The following packaging configurations are available.

Storage
- Store in refrigerator at 36°F to 46°F (2°C to 8°C) in its original carton to protect from light.
- Do not freeze or shake.
- For patients: If needed, store Palynziq in the original carton at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days. Record the date removed from refrigeration on the carton. Once stored at room temperature, do not return the product to the refrigerator.
- The shelf-life expires after storage at room temperature for 30 days, or after the expiration date on the product carton, whichever is earlier.
Images
Drug Images
{{#ask: Page Name::Pegvaliase |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel










{{#ask: Label Page::Pegvaliase |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling.
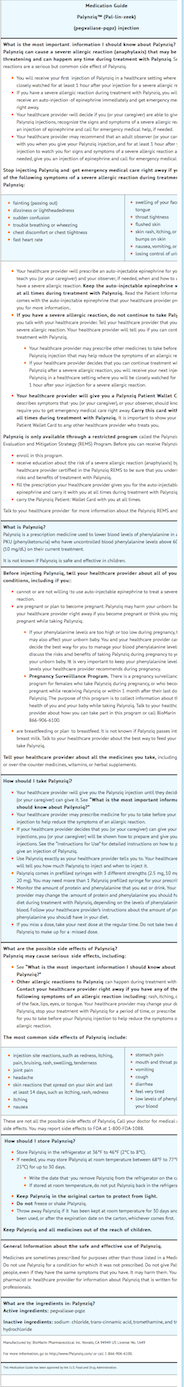
Anaphylaxis and Other Hypersensitivity Reactions
- Advise patients that Palynziq may cause hypersensitivity reactions, including anaphylaxis that can occur at any time. Instruct patients to recognize the signs and symptoms of anaphylaxis.
- Instruct patients to carry auto-injectable epinephrine with them at all times during Palynziq treatment. Instruct the patient and observer (if applicable) on the appropriate use of auto‑injectable epinephrine for anaphylaxis.
- Instruct patients who experience anaphylaxis to seek immediate medical care, discontinue therapy, and resume treatment only at the instruction of a healthcare provider.
Palynziq REMS Program
- Palynziq is available only through a restricted program called the Palynziq REMS. Inform the patient of the following notable requirements:
- Patients must be enrolled in the Palynziq REMS.
- Patients must be educated about the risk of anaphylaxis by a certified prescriber to ensure they understand the risks and benefits of treatment with Palynziq.
- Patients must fill a prescription for auto-injectable epinephrine and carry it with them at all times.
- Patients will be given a Palynziq Patient Wallet Card that they should carry with them at all times. This card describes symptoms which, if experienced, should prompt the :*patient and observer (if applicable) to immediately seek medical care. Advise the patient to show the Palynziq Wallet Card to other treating healthcare providers.
- Palynziq is available only from certified pharmacies participating in the program. Therefore, provide patients with the telephone number and website for information on how to obtain the product.
Administration
- Advise patients to monitor their dietary protein and phenylalanine intake throughout treatment with Palynziq, and adjust intake as directed by their healthcare provider based on blood phenylalanine concentrations.
- Provide appropriate instruction for methods of self-injection, including careful review of the Palynziq Medication Guide and Instructions for Use. Instruct patients in the use of aseptic technique when administering Palynziq.
- Inform patients that a healthcare provider will show them or their caregiver how to prepare to inject Palynziq before self-administering.
- Advise patients not to inject into moles, scars, birthmarks, bruises, rashes, or areas where the skin is hard, tender, red, damaged, burned, inflamed, or tattooed.
- Advise patients to rotate areas of injection with each dose. Advise patients to check the injection site for redness, swelling, and tenderness, and to contact their healthcare provider if they have a skin reaction and it does not clear up, or worsens.
- Advise patients to follow sharps disposal recommendations patients on safe disposal procedures.
- Advise patients that the shelf‑life expires after storage at room temperature for 30 days or after the expiration date on the product carton, whichever is earlier.
Pregnancy
- Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy.
- Advise women who are exposed to Palynziq during pregnancy or who become pregnant within one month following the last dose of Palynziq that there is a pregnancy surveillance program that monitors pregnancy outcomes. Encourage these patients to report their pregnancy to BioMarin (1‑866‑906‑6100).
Precautions with Alcohol
Alcohol-Pegvaliase interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Palynziq
Look-Alike Drug Names
There is limited information regarding Pegvaliase Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.