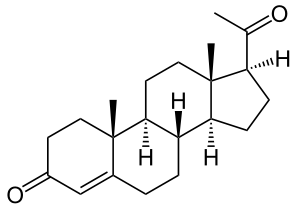
Progesterone (vaginal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Progesterone (vaginal) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Endometrin is indicated to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an Assisted Reproductive Technology (ART) treatment program for infertile women.
Dosage
2.1 General Dosing Information The dose of Endometrin is 100 mg administered vaginally two or three times daily starting the day after oocyte retrieval and continuing for up to 10 weeks total duration. Efficacy in women 35 years of age and older has not been clearly established. The appropriate dose of Endometrin in this age group has not been determined.
3 DOSAGE FORMS AND STRENGTHS 100 mg vaginal insert is a white to off-white oblong-shaped tablet debossed with "FPI" on one side and "100" on the other side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Progesterone (vaginal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Progesterone (vaginal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Progesterone (vaginal) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Progesterone (vaginal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Progesterone (vaginal) in pediatric patients.
Contraindications
Endometrin should not be used in individuals with any of the following conditions:
Previous allergic reactions to progesterone or any of the ingredients of Endometrin [see DESCRIPTION (11)] Known missed abortion or ectopic pregnancy Liver disease Known or suspected breast cancer Active arterial or venous thromboembolism or severe thrombophlebitis, or a history of these events
Warnings
5.1 Cardiovascular or Cerebrovascular Disorders The physician should be alert to earliest signs of myocardial infarction, cerebrovascular disorders, arterial or venous thromboembolism (venous thromboembolism or pulmonary embolism), thrombophlebitis, or retinal thrombosis. Endometrin should be discontinued if any of these are suspected.
5.2 Depression Patients with a history of depression need to be closely observed. Consider discontinuation if symptoms worsen.
5.3 Use of Other Vaginal Products Endometrin should not be recommended for use with other vaginal products (such as antifungal products as this may alter progesterone release and absorption from the vaginal insert.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data reflect exposure to Endometrin in 808 infertile women (74.9% White, 10.3% Hispanic, 5.4% Black, 5% Asian, and 4.6% Other) in a single Assisted Reproductive Technology 10 week clinical study conducted in the U.S. Endometrin was studied at doses of 100 mg twice daily and 100 mg three times daily. The adverse reactions that occurred at a rate greater than or equal to 2% in either Endometrin group are summarized in Table 1.

Other less common reported adverse reactions included vaginal irritation, itching, burning, discomfort, urticaria, and peripheral edema.
6.2 Expected Adverse Reaction Profile Seen with Progesterone Endometrin is also expected to have adverse reactions similar to other drugs containing progesterone that may include breast tenderness, bloating, mood swings, irritability, and drowsiness.
Postmarketing Experience
There is limited information regarding Progesterone (vaginal) Postmarketing Experience in the drug label.
Drug Interactions
No formal drug-drug interaction studies have been conducted for Endometrin. Drugs known to induce the hepatic cytochrome-P450-3A4 system (such as rifampin, carbamazepine) may increase the elimination of progesterone. The effect of concomitant vaginal products on the exposure of progesterone from Endometrin has not been assessed. Endometrin is not recommended for use with other vaginal products (such as antifungal products) as this may alter progesterone release and absorption from the vaginal insert.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Endometrin has been used to support embryo implantation and maintain clinical pregnancy in one clinical study. The livebirth outcomes of these pregnancies were as follows:
Among the 404 subjects treated with Endometrin twice daily, 143 subjects had livebirths consisting of 85 singletons, 56 twins, and 2 triplets. In this treatment group, 13 subjects had a spontaneous abortion, 1 subject had an ectopic pregnancy, and 7 subjects reported fetal birth defects (3.4% based on 203 livebirths). Among the 404 subjects treated with Endometrin three times daily, 155 subjects had livebirths consisting of 91 singletons, 60 twins, and 4 triplets. In this treatment group, 22 subjects had a spontaneous abortion, 4 subjects had an ectopic pregnancy, and 7 subjects reported fetal birth defects (3.1% based on 223 livebirths). Birth defects reported in the Endometrin twice daily group included: one fetus with a cleft palate and intrauterine growth retardation, one fetus with spina bifida, three fetuses with congenital heart defects, one fetus with an umbilical hernia, and one fetus with an intestinal anomaly.
Birth defects reported in the Endometrin three times daily group included: one fetus with an esophageal fistula, one fetus with hypospadias and an underdeveloped right ear, one fetus with Down's and an atrial septal defect, one fetus with congenital heart anomalies, one fetus with DiGeorge's syndrome, one fetus with a hand deformity, and one fetus with cleft palate.
For additional information on the pharmacology of Endometrin and pregnancy outcome information
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Progesterone (vaginal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Progesterone (vaginal) during labor and delivery.
Nursing Mothers
Detectable amounts of progesterone have been identified in the milk of nursing mothers. The effect of this on the nursing infant has not been determined.
Pediatric Use
This drug is not intended for pediatric use and no clinical data have been collected in children. Therefore, the safety and effectiveness of Endometrin in pediatric patients have not been established.
Geriatic Use
No clinical data have been collected in patients over age 65.
Gender
There is no FDA guidance on the use of Progesterone (vaginal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Progesterone (vaginal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Progesterone (vaginal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Progesterone (vaginal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Progesterone (vaginal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Progesterone (vaginal) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Progesterone (vaginal) Administration in the drug label.
Monitoring
There is limited information regarding Progesterone (vaginal) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Progesterone (vaginal) and IV administrations.
Overdosage
Treatment of overdosage consists of discontinuation of Endometrin together with institution of appropriate symptomatic and supportive care.
Pharmacology
Mechanism of Action
Progesterone is a naturally occurring steroid that is secreted by the ovary, placenta, and adrenal gland. In the presence of adequate estrogen, progesterone transforms a proliferative endometrium into a secretory endometrium. Progesterone is necessary to increase endometrial receptivity for implantation of an embryo. Once an embryo is implanted, progesterone acts to maintain a pregnancy.
Structure
Endometrin (progesterone) Vaginal Insert contains micronized progesterone. Endometrin is supplied with polyethylene vaginal applicators.
The active ingredient, progesterone, is present in 100 mg amount along with other excipients. The chemical name for progesterone is pregn-4-ene-3,20-dione. It has an empirical formula of C21H30O2 and a molecular weight of 314.5. Progesterone exists in two polymorphic forms. The form used in Endometrin, the alpha-form, has a melting point of 127-131°C.
The structural formula is:
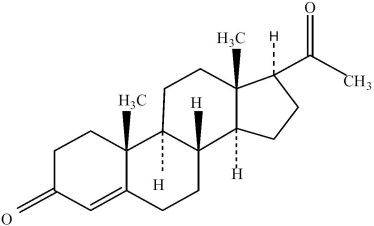
Each Endometrin Vaginal Insert delivers 100 mg of progesterone in a base containing lactose monohydrate, polyvinylpyrrolidone, adipic acid, sodium bicarbonate, sodium lauryl sulfate, magnesium stearate, pregelatinized starch, and colloidal silicon dioxide.
Pharmacodynamics
There is limited information regarding Progesterone (vaginal) Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
Progesterone serum concentrations increased following the administration of the Endometrin Vaginal Insert in 12 healthy pre-menopausal females. On single dosing, the mean Cmax was 17.0 ng/mL in the Endometrin twice daily group and 19.8 ng/mL in the Endometrin three times daily group. On multiple dosing, steady-state concentrations were attained within approximately 1 day after initiation of treatment with Endometrin. Both Endometrin regimens provided average serum concentrations of progesterone exceeding 10 ng/mL on Day 5. The pharmacokinetic results are summarized in Table 2.

Distribution
Progesterone is approximately 96% to 99% bound to serum proteins, primarily to serum albumin and corticosteroid binding globulin.
Metabolism
Progesterone is metabolized primarily by the liver largely to pregnanediols and pregnanolones. Pregnanediols and pregnanolones are conjugated in the liver to glucuronide and sulfate metabolites. Progesterone metabolites that are excreted in the bile may be deconjugated and may be further metabolized in the gut via reduction, dehydroxylation, and epimerization.
Excretion
Progesterone undergoes renal and biliary elimination. Following injection of labeled progesterone, 50-60% of the excretion of metabolites occurs via the kidney; approximately 10% occurs via the bile and feces. Overall recovery of the labeled material accounts for 70% of an administered dose. Only a small portion of unchanged progesterone is excreted in the bile.
Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Nonclinical toxicity studies to determine the potential of Endometrin to cause carcinogenicity or mutagenicity have not been performed. The effect of Endometrin on fertility has not been evaluated in animals.
Clinical Studies
14.1 Luteal Supplementation During Assisted Reproductive Treatment Study A randomized, open-label, active-controlled study evaluated the efficacy of 10 weeks of treatment with two different daily dosing regimens of Endometrin (100 mg twice daily and 100 mg three times daily) for support of implantation and early pregnancy in infertile women participating in an Assisted Reproductive Technology treatment program. Efficacy was assessed on the endpoint of ongoing pregnancies, defined as the presence of at least one fetal heartbeat seen on ultrasound at 6 weeks post-embryo transfer. The study randomized to Endometrin 808 infertile women (74.9% White; 10.3% Hispanic, 5.4% Black, 5% Asian, and 4.6% Other) between 19 and 42 years of age (mean age 33) who had a body mass index <34 kg/m2 at screening.
The ongoing pregnancy rates for subjects treated with both dosing regimens of Endometrin were noninferior (lower bounds of the 95% confidence interval of the difference between Endometrin and the active comparator excluded a difference greater than 10%) to the ongoing pregnancy rate for subjects treated with the active comparator. The results of this study are shown in Table 3.

Subjects participating in the study were stratified at randomization by age and ovarian reserve (as measured by serum FSH levels). The ongoing pregnancy rates for these subgroups are shown in Table 4.

In subjects under the age of 35 or with serum FSH levels less than 10 IU/L, results from both dosing regimens were non-inferior to the results from the comparator with respect to ongoing pregnancy rates. In women age 35 and older and in women with serum FSH levels between 10 and 15 IU/L, the results with respect to ongoing pregnancy rates for both dosing regimens of Endometrin did not reach the criteria for non-inferiority.
Subjects who became pregnant received study medication for a total of 10 weeks. Patients over 34 kg/m2 were not studied. The efficacy of Endometrin in this patient group is unknown
How Supplied
Each Endometrin Vaginal Insert is a white to off-white oblong-shaped insert debossed with "FPI" on one side and "100" on the other side. Each Endometrin® (progesterone) Vaginal Insert, 100 mg, is packed individually in a sealed foil pouch. These pouches are available in cartons packed:
21 vaginal inserts with 21 disposable vaginal applicators (NDC 55566-6500-3)
Storage
Store at 20 - 25°C (68 - 77°F); excursions permitted between 15 - 30°C (59 - 86°F).
Images
Drug Images
{{#ask: Page Name::Progesterone (vaginal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Progesterone (vaginal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
17.1 Vaginal Bleeding Inform patients of the importance of reporting irregular vaginal bleeding to their doctor as soon as possible.
17.2 Common Adverse Reactions with Progesterone Inform patients of the possible side effects of progesterone therapy such as headaches, breast tenderness, bloating, mood swings, irritability, and drowsiness.
17.3 Coadministration of Vaginal Products Inform patients that Endometrin is not recommended for use with other vaginal products.
Patient Package Insert

Precautions with Alcohol
Alcohol-Progesterone (vaginal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Progesterone (vaginal) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Progesterone (vaginal) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.