Ganciclovir (ophthalmic)
{{DrugProjectFormSinglePage |authorTag=Aparna Vuppala, M.B.B.S. [1]
|genericName=
|aOrAn=
a
|drugClass=
|indication=
|hasBlackBoxWarning=
Yes
|adverseReactions=
|blackBoxWarningTitle=
Title
|blackBoxWarningBody= ConditionName:
- Content
|fdaLIADAdult=
- ZIRGAN (ganciclovir ophthalmic gel) 0.15% is indicated for the treatment of acute herpetic keratitis (dendritic ulcers)
Dosage
- The recommended dosing regimen for ZIRGAN is 1 drop in the affected eye 5 times per day (approximately every 3 hours while awake) until the corneal ulcer heals, and then 1 drop 3 times per day for 7 days.
|offLabelAdultGuideSupport=
There is limited information regarding Off-Label Guideline-Supported Use of Ganciclovir (ophthalmic) in adult patients.
|offLabelAdultNoGuideSupport=
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ganciclovir (ophthalmic) in adult patients.
|fdaLIADPed=
There is limited information regarding FDA-Labeled Use of Ganciclovir (ophthalmic) in pediatric patients.
|offLabelPedGuideSupport=
There is limited information regarding Off-Label Guideline-Supported Use of Ganciclovir (ophthalmic) in pediatric patients.
|offLabelPedNoGuideSupport=
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ganciclovir (ophthalmic) in pediatric patients.
|contraindications=
- None.
|warnings=
- Topical Ophthalmic Use Only
- ZIRGAN is indicated for topical ophthalmic use only.
- Avoidance of Contact Lenses
- Patients should not wear contact lenses if they have signs or symptoms of herpetic keratitis or during the course of therapy with ZIRGAN.
|clinicalTrials=
- Most common adverse reactions reported in patients were blurred vision (60%), eye irritation (20%), punctate keratitis (5%), and conjunctival hyperemia (5%).
|postmarketing=
There is limited information regarding Postmarketing Experience of Ganciclovir (ophthalmic) in the drug label.
|drugInteractions=
|useInPregnancyFDA=
- Pregnancy Category C. Ganciclovir has been shown to be embryotoxic in rabbits and mice following intravenous administration and teratogenic in rabbits. Fetal resorptions were present in at least 85% of rabbits and mice administered 60 mg/kg/day and 108 mg/kg/day (approximately 10,000x and 17,000x the human ocular dose of 6.25 mcg/kg/day), respectively, assuming complete absorption. Effects observed in rabbits included: fetal growth retardation, embryolethality, teratogenicity, and/or maternal toxicity. Teratogenic changes included cleft palate, anophthalmia/microphthalmia, aplastic organs (kidney and pancreas), hydrocephaly, and brachygnathia. In mice, effects observed were maternal/fetal toxicity and embryolethality. Daily intravenous doses of 90 mg/kg/day (14,000x the human ocular dose) administered to female mice prior to mating, during gestation, and during lactation caused hypoplasia of the testes and seminal vesicles in the month-old male offspring, as well as pathologic changes in the nonglandular region of the stomach
- There are no adequate and well-controlled studies in pregnant women. ZIRGAN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
|useInPregnancyAUS=
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ganciclovir (ophthalmic) in women who are pregnant.
|useInLaborDelivery=There is no FDA guidance on use of Ganciclovir (ophthalmic) during labor and delivery.
|useInNursing=
- It is not known whether topical ophthalmic ganciclovir administration could result in sufficient systemic absorption to produce detectable quantities in breast milk. Caution should be exercised when ZIRGAN is administered to nursing mothers.
|useInPed=
- Safety and efficacy in pediatric patients below the age of 2 years have not been established.
|useInGeri=
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
|useInGender= There is no FDA guidance on the use of Ganciclovir (ophthalmic) with respect to specific gender populations.
|useInRace= There is no FDA guidance on the use of Ganciclovir (ophthalmic) with respect to specific racial populations.
|useInRenalImpair= There is no FDA guidance on the use of Ganciclovir (ophthalmic) in patients with renal impairment.
|useInHepaticImpair= There is no FDA guidance on the use of Ganciclovir (ophthalmic) in patients with hepatic impairment.
|useInReproPotential= There is no FDA guidance on the use of Ganciclovir (ophthalmic) in women of reproductive potentials and males.
|useInImmunocomp= There is no FDA guidance one the use of Ganciclovir (ophthalmic) in patients who are immunocompromised.
|administration=
- Ophthalmic
|monitoring=
There is limited information regarding Monitoring of Ganciclovir (ophthalmic) in the drug label.
|IVCompat=
There is limited information regarding IV Compatibility of Ganciclovir (ophthalmic) in the drug label.
|overdose=
There is limited information regarding Chronic Overdose of Ganciclovir (ophthalmic) in the drug label.
|drugBox=
|mechAction=
- ZIRGAN (ganciclovir ophthalmic gel) 0.15% contains the active ingredient, ganciclovir, which is a guanosine derivative that, upon phosphorylation, inhibits DNA replication by herpes simplex viruses (HSV). Ganciclovir is transformed by viral and cellular thymidine kinases (TK) to ganciclovir triphosphate, which works as an antiviral agent by inhibiting the synthesis of viral DNA in 2 ways: competitive inhibition of viral DNA-polymerase and direct incorporation into viral primer strand DNA, resulting in DNA chain termination and prevention of replication.
|structure=
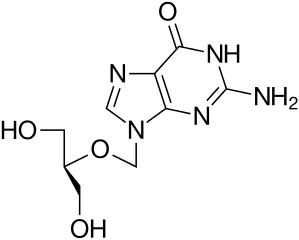
- ZIRGAN (ganciclovir ophthalmic gel) 0.15% contains a sterile, topical antiviral for ophthalmic use. The chemical name is 9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]guanine (CAS number 82410-32-0). Ganciclovir is represented by the following structural formula:
- Ganciclovir has a molecular weight of 255.23, and the empirical formula is C9H13N5O4.
- Each gram of gel contains: ACTIVE: ganciclovir 1.5 mg (0.15%). INACTIVES: Carbomer Homopolymer, water for injection, sodium hydroxide (to adjust the pH to 7.4), mannitol. PRESERVATIVE: benzalkonium chloride 0.075 mg.
|PD=
There is limited information regarding Pharmacodynamics of Ganciclovir (ophthalmic) in the drug label.
|PK=
- The estimated maximum daily dose of ganciclovir administered as 1 drop, 5 times per day is 0.375 mg. Compared to maintenance doses of systemically administered ganciclovir of 900 mg (oral valganciclovir) and 5 mg/kg (IV ganciclovir), the ophthalmically administered daily dose is approximately 0.04% and 0.1% of the oral dose and IV doses, respectively, thus minimal systemic exposure is expected.
|nonClinToxic=
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Ganciclovir was carcinogenic in the mouse at oral doses of 20 and 1,000 mg/kg/day (approximately 3,000x and 160,000x the human ocular dose of 6.25 mcg/kg/day, assuming complete absorption). At the dose of 1,000 mg/kg/day there was a significant increase in the incidence of tumors of the preputial gland in males, forestomach (nonglandular mucosa) in males and females, and reproductive tissues (ovaries, uterus, mammary gland, clitoral gland, and vagina) and liver in females. At the dose of 20 mg/kg/day, a slightly increased incidence of tumors was noted in the preputial and harderian glands in males, forestomach in males and females, and liver in females. No carcinogenic effect was observed in mice administered ganciclovir at 1 mg/kg/day (160x the human ocular dose). Except for histocytic sarcoma of the liver, ganciclovir-induced tumors were generally of epithelial or vascular origin. Although the preputial and clitoral glands, forestomach and harderian glands of mice do not have human counterparts, ganciclovir should be considered a potential carcinogen in humans. Ganciclovir increased mutations in mouse lymphoma cells and DNA damage in human lymphocytes in vitro at concentrations between 50 to 500 and 250 to 2,000 mcg/mL, respectively.
- In the mouse micronucleus assay, ganciclovir was clastogenic at doses of 150 and 500 mg/kg (IV) (24,000x to 80,000x human ocular dose) but not 50 mg/kg (8,000x human ocular dose). Ganciclovir was not mutagenic in the Ames Salmonella assay at concentrations of 500 to 5,000 mcg/mL.
- Ganciclovir caused decreased mating behavior, decreased fertility, and an increased incidence of embryolethality in female mice following intravenous doses of 90 mg/kg/day (approximately 14,000x the human ocular dose of 6.25 mcg/kg/day). Ganciclovir caused decreased fertility in male mice and hypospermatogenesis in mice and dogs following daily oral or intravenous administration of doses ranging from 0.2 to 10 mg/kg (30x to 1,600x the human ocular dose).
|clinicalStudies=
- In one open-label, randomized, controlled, multicenter clinical trial which enrolled 164 patients with herpetic keratitis, ZIRGAN was non-inferior to acyclovir ophthalmic ointment, 3% in patients with dendritic ulcers. Clinical resolution (healed ulcers) at Day 7 was achieved in 77% (55/71) for ZIRGAN versus 72% (48/67) for acyclovir 3% (difference 5.8%, 95% CI -9.6%-18.3%). In three randomized, single-masked, controlled, multicenter clinical trials which enrolled 213 total patients, ZIRGAN was non-inferior to acyclovir ophthalmic ointment 3% in patients with dendritic ulcers. Clinical resolution at Day 7 was achieved in 72% (41/57) for ZIRGAN versus 69% (34/49) for acyclovir (difference 2.5%, 95% CI -15.6%-20.9%).
|howSupplied=
- ZIRGAN is supplied as 5 grams of a sterile, preserved, clear, colorless, topical ophthalmic gel containing O.15% of ganciclovir in a polycoated aluminum tube with a white polyethylene tip and cap and protective band (NDC 24208-535-35).
- Storage
- Store at 15°C-25°C (59°F-77°F). Do not freeze.
|fdaPatientInfo=
- This product is sterile when packaged. Patients should be advised not to allow the dropper tip to touch any surface, as this may contaminate the gel. If pain develops, or if redness, itching, or inflammation becomes aggravated, the patient should be advised to consult a physician. Patients should be advised not to wear contact lenses when using ZIRGAN.
|alcohol=
- Alcohol-Ganciclovir (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=
|lookAlike=
|drugShortage= }}
{{#subobject:
|Label Page=Ganciclovir (ophthalmic) |Label Name=Ganciclovir (ophthalmic)06.png
}}
{{#subobject:
|Label Page=Ganciclovir (ophthalmic) |Label Name=Ganciclovir (ophthalmic)07.png
}}