Topotecan (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Topotecan (oral) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
HYCAMTIN capsules are indicated for the treatment of relapsed small cell lung cancer in patients with a prior complete or partial response and who are at least 45 days from the end of first-line chemotherapy.
Dosage
2.1 Recommended Dosing The recommended dose of HYCAMTIN capsules is 2.3 mg/m2/day orally once daily for 5 consecutive days repeated every 21 days. Round the dose to the nearest 0.25 mg, and prescribe the minimum number of 1-mg and 0.25-mg capsules. Prescribe the same number of capsules for each of the 5 dosing days.
Take HYCAMTIN capsules with or without food. Swallow capsules whole. Do not chew, crush, or divide the capsules. Do not prescribe a replacement dose for emesis.
Diarrhea:
Do not administer HYCAMTIN capsules to patients with Grade 3 or 4 diarrhea. After recovery to Grade 1 or less, reduce the dose of HYCAMTIN by 0.4 mg/m2/day for subsequent courses [see Warnings and Precautions (5.2)].
2.2 Dose Modification Guidelines Hematologic Toxicities:
Do not administer subsequent courses of HYCAMTIN capsules until neutrophils recover to greater than 1,000 cells/mm3, platelets recover to greater than 100,000 cells/mm3, hemoglobin levels recover to greater than or equal to 9.0 g/dL (with transfusion if necessary). Renal Impairment: The recommended starting doses of HYCAMTIN capsules in patients with moderate and severe renal impairment are as follows: Dose reduce HYCAMTIN capsules by 0.4 mg/m2/day for: neutrophil counts of less than 500 cells/mm3 associated with fever or infection or lasting for 7 days or more; neutrophil counts of 500 to 1,000 cells/mm3 lasting beyond day 21 of the treatment course; platelet counts less than 25,000 cells/mm3. Renal Impairment:
The recommended starting doses of HYCAMTIN capsules in patients with moderate and severe renal impairment are as follows:

Dosage Forms and Strengths
- HYCAMTIN capsules contain topotecan hydrochloride expressed as topotecan free base. The 0.25-mg capsules are opaque white to yellowish-white and imprinted with HYCAMTIN and 0.25 mg. The 1-mg capsules are opaque pink and imprinted with HYCAMTIN and 1 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Topotecan (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Topotecan (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Topotecan (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Topotecan (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Topotecan (oral) in pediatric patients.
Contraindications
HYCAMTIN is contraindicated in patients who have a history of severe hypersensitivity reactions to topotecan.
Warnings
5.1 Bone Marrow Suppression Bone marrow suppression (primarily neutropenia) is a dose-limiting toxicity of HYCAMTIN. Neutropenia is not cumulative over time. The following data on myelosuppression are based on an integrated safety database from 4 thoracic malignancy trials (N = 682) using HYCAMTIN capsules at 2.3 mg/m2/day for 5 consecutive days. The median day for neutrophil and platelet nadirs occurred on Day 15.
Neutropenia:
Grade 4 neutropenia (<500 cells/mm3) occurred in 32% of patients with a median duration of 7 days and was most common during Course 1 of treatment (20% of patients). Clinical sequelae of neutropenia included infection (17%), febrile neutropenia (4%), sepsis (2%), and septic death (1%). Pancytopenia has been reported.
Topotecan can cause fatal typhlitis (neutropenic enterocolitis). Consider the possibility of typhlitis in patients presenting with fever, neutropenia, and abdominal pain [see Dosage and Administration (2.2)].
Thrombocytopenia:
Grade 4 thrombocytopenia (<10,000 cells/mm3) occurred in 6% of patients, with a median duration of 3 days.
Anemia:
Grade 3 or 4 anemia (<8 g/dL) occurred in 25% of patients.
Administer the first course of HYCAMTIN only to patients with a neutrophil count of ≥1,500 cells/mm3 and a platelet count ≥100,000 cells/mm3. Monitor peripheral blood cell counts frequently during treatment with HYCAMTIN. Refer to Section 2.2 for dose modification guidelines for hematological toxicities in subsequent courses.
5.2 Diarrhea Diarrhea, including severe and life-threatening diarrhea requiring hospitalization, can occur during treatment with HYCAMTIN capsules. Diarrhea caused by HYCAMTIN capsules can occur at the same time as drug-induced neutropenia and its sequelae. In the 682 patients who received HYCAMTIN capsules in the 4 lung cancer trials, the incidence of diarrhea caused by HYCAMTIN capsules was 22%, with 4% Grade 3 and 0.4% Grade 4.The incidence of Grade 3 or 4 diarrhea proximate (within 5 days) to Grade 3 or 4 neutropenia events in the group receiving HYCAMTIN capsules was 5%. The median time to onset of Grade 2 or worse diarrhea was 9 days in the group receiving HYCAMTIN capsules. Manage diarrhea caused by HYCAMTIN capsules aggressively. Do not administer HYCAMTIN capsules to patients with Grade 3 or 4 diarrhea. Reduce the dose of HYCAMTIN after recovery to Grade 1 or less [see Dosage and Administration (2.2)].
5.3 Interstitial Lung Disease Interstitial lung disease (ILD), including fatalities, has occurred with HYCAMTIN. Underlying risk factors include history of ILD, pulmonary fibrosis, lung cancer, thoracic radiation, and use of pneumotoxic drugs and/or colony stimulating factors. Monitor patients for pulmonary symptoms indicative of interstitial lung disease (e.g., cough, fever, dyspnea, and/or hypoxia), and discontinue HYCAMTIN if a new diagnosis of ILD is confirmed.
5.4 Embryofetal Toxicity HYCAMTIN can cause fetal harm when administered to a pregnant woman. Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis. If this drug is used during pregnancy, or if a patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [see Use in Specific Populations (8.1)].
Advise females of reproductive potential to use highly effective contraception during treatment and for at least 1 month after the last dose of HYCAMTIN. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking HYCAMTIN [see Use in Specific Populations (8.1, 8.7)].
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of HYCAMTIN capsules was evaluated in 682 patients with lung cancer (3 recurrent small cell lung cancer [SCLC] trials and 1 recurrent non-small cell lung cancer [NSCLC] trial) who received at least one dose of HYCAMTIN capsules. Patients in all four trials had advanced lung malignancies and received prior chemotherapy in the first-line setting. The dose regimen for HYCAMTIN capsules was 2.3 mg/m2/day for five consecutive days every 21 days. The median number of courses was 3 (range: 1 to 20) in these four trials. Table 2 describes the hematologic and non-hematologic adverse reactions in recurrent SCLC patients treated with HYCAMTIN capsules in the overall lung cancer patient population.

On-Study Death Due to Toxicity of HYCAMTIN: In the 682 patients who received HYCAMTIN capsules in the four lung cancer trials, 39 deaths (6%) occurred within 30 days after the last dose for a reason other than progressive disease: 13 due to hematologic toxicity, 5 due to non-hematologic toxicity (2 from diarrhea), and 21 due to other causes.
Postmarketing Experience
There is limited information regarding Topotecan (oral) Postmarketing Experience in the drug label.
Drug Interactions
Topotecan is a substrate for both P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP). Inhibitors of these transporters increase the systemic exposure to oral topotecan. Avoid concomitant use of P-gp inhibitors (e.g., amiodarone, azithromycin, captopril, carvedilol, clarithromycin, conivaptan, cyclosporine, diltiazem, dronedarone, erythromycin, felodipine, itraconazole, ketoconazole, lopinavir, ritonavir, quercetin, quinidine, ranolazine, ticagrelor, verapamil) and BCRP inhibitors (e.g., cyclosporine, eltrombopag) with HYCAMTIN capsules [see Clinical Pharmacology (12.3)].
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D Risk Summary:
HYCAMTIN can cause fetal harm when administered to a pregnant woman. Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, inform the patient of the potential hazard to a fetus.
Animal Data:
In rabbits, an IV dose of 0.10 mg/kg/day (about equal to the clinical IV dose on a mg/m2 basis) given on days 6 through 20 of gestation caused maternal toxicity, embryolethality, and reduced fetal body weight. In the rat, an IV dose of 0.23 mg/kg/day (about equal to the clinical IV dose on a mg/m2 basis) given for 14 days before mating through gestation day 6 caused fetal resorption, microphthalmia, pre-implant loss, and mild maternal toxicity. Administration of an IV dose of 0.10 mg/kg/day (about half the clinical IV dose on a mg/m2 basis) to rats on days 6 through 17 of gestation caused an increase in post-implantation mortality. This dose also caused an increase in total fetal malformations. The most frequent malformations were of the eye (microphthalmia, anophthalmia, rosette formation of the retina, coloboma of the retina, ectopic orbit), brain (dilated lateral and third ventricles), skull, and vertebrae.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Topotecan (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Topotecan (oral) during labor and delivery.
Nursing Mothers
It is not known whether topotecan is present in human milk. Lactating rats excrete high concentrations of topotecan into milk. Female rats given 4.72 mg/m2 IV (about twice the clinical dose on a mg/m2 basis) excreted topotecan into milk at concentrations up to 48-fold higher than those in plasma. Because many drugs are present in human milk, and because of the potential for serious adverse reactions in nursing infants from HYCAMTIN, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Of the 682 patients with thoracic cancer in 4 clinical trials who received HYCAMTIN capsules, 33% (n = 225) were aged 65 years and older, while 4.8% (n = 33) were aged 75 years and older. Treatment-related diarrhea was more frequent in patients aged ≥65 years (28%) compared with those younger than 65 years (19%). [See Warnings and Precautions (5.2), Adverse Reactions (6.1).]
No overall differences in effectiveness were observed between patients 65 years and older and younger patients.
Gender
There is no FDA guidance on the use of Topotecan (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Topotecan (oral) with respect to specific racial populations.
Renal Impairment
The systemic exposure to both topotecan lactone and total topotecan increased in patients with renal impairment compared with that in patients with normal renal function. No dosage adjustment is recommended for patients with mild renal impairment (CLcr = 50-79 mL/min). Adjust the dose of HYCAMTINcapsules in patients with moderate (CLcr = 30-49 mL/min) and severe (CLcr <30 mL/min) renal impairment[see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
Hepatic Impairment
There is no FDA guidance on the use of Topotecan (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
Contraception:
Females: Counsel patients on pregnancy planning and prevention. Advise female patients of reproductive potential to use highly effective contraception during and for 1 month following treatment with HYCAMTIN. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking HYCAMTIN [see Use in Specific Populations (8.1)].
Males: HYCAMTIN may damage spermatozoa, resulting in possible genetic and fetal abnormalities. Advise males with a female sexual partner of reproductive potential to use effective contraception during and for 3 months after treatment with HYCAMTIN [see Nonclinical Toxicology (13.1)].
Infertility:
Females: In females of reproductive potential, HYCAMTIN may have both acute and long-term effects on fertility [see Nonclinical Toxicology (13.1)].
Males: Effects on spermatogenesis have been observed in animals administered HYCAMTIN. Advise males of the potential risk for impaired fertility and to seek counseling on fertility and family planning options prior to starting treatment.
Immunocompromised Patients
There is no FDA guidance one the use of Topotecan (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Topotecan (oral) Administration in the drug label.
Monitoring
There is limited information regarding Topotecan (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Topotecan (oral) and IV administrations.
Overdosage
Overdoses (up to 5-fold of the prescribed dose) occurred in patients treated with HYCAMTIN capsules. The primary complication of overdosage is bone marrow suppression. The observed signs and symptoms of overdose are consistent with the known adverse reactions associated with HYCAMTIN for oral use [see Adverse Reactions (6.1)]. Mucositis has also been reported in association with overdose.
There is no known antidote for overdosage with HYCAMTIN. If an overdose is suspected, monitor the patient closely for bone marrow suppression, and institute supportive-care measures (such as the prophylactic use of G-CSF and/or antibiotic therapy) as appropriate.
Pharmacology
Mechanism of Action
Topoisomerase I relieves torsional strain in DNA by inducing reversible single-strand breaks. Topotecan binds to the topoisomerase I-DNA complex and prevents re-ligation of these single strand breaks. The cytotoxicity of topotecan is thought to be due to double-strand DNA damage produced during DNA synthesis, when replication enzymes interact with the ternary complex formed by topotecan, topoisomerase I, and DNA. Mammalian cells cannot efficiently repair these double-strand breaks.
Structure
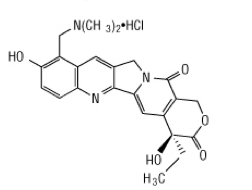
Topotecan hydrochloride is a semi-synthetic derivative of camptothecin and is an anti-tumor drug with topoisomerase I-inhibitory activity.
The chemical name for topotecan hydrochloride is (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7] indolizino [1,2-b]quinoline-3,14-(4H,12H)-dione monohydrochloride. It has the molecular formula C23H23N3O5•HCl and a molecular weight of 457.9. It is soluble in water and melts with decomposition at 213° to 218°C.
Topotecan hydrochloride has the following structural formula:
HYCAMTIN capsules for oral use contain topotecan hydrochloride, the content of which is expressed as topotecan free base. The excipients are gelatin, glyceryl monostearate, hydrogenated vegetable oil, and titanium dioxide. The capsules are imprinted with edible black ink. The 1-mg capsules also contain red iron oxide.
Pharmacodynamics
There is limited information regarding Topotecan (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
Following administration of HYCAMTIN capsules at doses of 1.2 to 3.1 mg/m2 administered daily for 5 days in cancer patients, topotecan exhibited biexponential pharmacokinetics with a mean terminal half-life of 3 to 6 hours. Total exposure (AUC) increased approximately proportionally to dose.
Absorption:
Topotecan is rapidly absorbed with peak plasma concentrations occurring between 1 to 2 hours following oral administration. The oral bioavailability of topotecan is approximately 40%. Following a high-fat meal, the extent of exposure was similar in the fed and fasted states, while Tmax was delayed from 1.5 to 3 hours for topotecan lactone and from 3 to 4 hours for total topotecan. HYCAMTIN capsules can be given without regard to food.
Distribution:
Binding of topotecan to plasma proteins is approximately 35%.
Metabolism:
Topotecan undergoes a reversible pH-dependent hydrolysis of its lactone moiety; it is the lactone form that is pharmacologically active. At pH ≤4, the lactone is exclusively present, whereas the ring-opened hydroxy-acid form predominates at physiologic pH. The mean metabolite:parent AUC ratio was <10% for total topotecan and topotecan lactone.
Excretion:
In a mass balance study in 4 patients with advanced solid tumors, the overall recovery of drug-related material following 5 daily doses of topotecan was 57% of the administered oral dose. In the urine, 20% of the orally administered dose was excreted as total topotecan and 2% was excreted as N-desmethyl topotecan [see Use in Specific Populations (8.6)].
Fecal elimination of total topotecan accounted for 33%, while fecal elimination of N-desmethyl topotecan was 1.5%. Overall, the N-desmethyl metabolite contributed a mean of <6% (range: 4% to 8%) of the total drug-related material accounted for in the urine and feces. O-glucuronides of both topotecan and N-desmethyl topotecan have been identified in the urine.
Specific Populations:
Age and Gender: A cross-study analysis in 217 patients with advanced solid tumors indicated that age and gender did not significantly affect the pharmacokinetics of oral topotecan.
Race: In patients with normal renal function, the exposures (geometric mean dose-normalized AUCinf) to topotecan lactone and total topotecan each were approximately 30% higher in Asian patients (n = 7) compared with Caucasian patients (n = 11).
In patients with mild renal impairment, the exposure was 30% higher for topotecan lactone in Asian (n = 7) compared with Caucasian (n = 12) patients, but the exposure to total topotecan was similar.
In patients with moderate renal impairment, the exposure was 60% higher for both topotecan lactone and total topotecan in Asian (n = 8) compared with Caucasian patients (n = 6).
In patients with severe renal impairment, the exposure was 112% higher for topotecan lactone and 70% higher for total topotecan in Asian (n = 3) compared with Caucasian patients (n = 4).
Renal Impairment: A trial was conducted in 59 patients with advanced cancer who were grouped based on the degree of their renal function and received HYCAMTIN capsules as shown in the table below.

The exposure (geometric mean dose-normalized AUCinf) for topotecan lactone increased by 34%, 80%, and 114% in Caucasian patients with mild, moderate, and severe renal impairment, respectively, compared with that in Caucasian patients with normal renal function. The corresponding values for total topotecan in Caucasian patients were 70%, 108%, and 227%, respectively. Asian patients with mild, moderate, and severe renal impairment had a 34%, 121%, and 247% higher exposure to topotecan lactone, respectively, than Asian patients with normal renal function. The corresponding values for total topotecan in Asian patients are 26%, 153%, and 331%, respectively. Prior platinum-based chemotherapy (P-B CT) had no effect on the systemic exposure to both total topotecan and topotecan lactone in patients with normal renal function.
No dosage adjustment is recommended for patients with mild renal impairment. Adjust the dosage of HYCAMTIN capsules in patients with moderate and severe renal impairment [see Dosage and Administration (2.2), Use in Specific Populations (8.6)].
Hepatic Impairment: In a population pharmacokinetic analysis involving oral topotecan administered at doses of 0.15 to 2.7 mg/m2/day to 118 cancer patients, the pharmacokinetics of total topotecan did not differ significantly based on patient serum bilirubin, ALT, or AST.
Drug Interactions:
Effects of Topotecan on Drug-Metabolizing Enzymes: In vitro inhibition studies using marker substrates known to be metabolized by human cytochromes P450 (CYP1A2, CYP2A6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E, CYP3A, or CYP4A) or dihydropyrimidine dehydrogenase indicate that the activities of these enzymes were not altered by topotecan. Enzyme inhibition by topotecan has not been evaluated in vivo.
Drugs That Inhibit Drug Efflux Transporters: Following coadministration of escalating doses of a dual inhibitor of BCRP and P-gp with oral topotecan, the AUCinf of topotecan lactone and total topotecan increased approximately 2.5-fold compared with control [see Drug Interactions (7.1)].
Administration of oral cyclosporine A (15 mg/kg), an inhibitor of P-gp, multidrug-resistance-associated protein (MRP-1), and cytochrome P450 3A4 (CYP3A4) within 4 hours of oral topotecan increased the dose-normalized AUC0-24h of topotecan lactone and total topotecan 2.0- to 3.0-fold compared with control [see Drug Interactions (7.1)].
Effect of pH-Elevating Agents: The pharmacokinetics of oral topotecan were unchanged when coadministered with ranitidine.
Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Carcinogenicity testing of topotecan has not been done. Nevertheless, topotecan is known to be genotoxic to mammalian cells and is a probable carcinogen. Topotecan was mutagenic to L5178Y mouse lymphoma cells and clastogenic to cultured human lymphocytes with and without metabolic activation. It was also clastogenic to mouse bone marrow. Topotecan did not cause mutations in bacterial cells.
Topotecan given to female rats prior to mating at a dose of 1.4 mg/m2 IV (about 0.6 times the oral clinical dose on a mg/m2 basis) caused superovulation possibly related to inhibition of follicular atresia. This dose given to pregnant female rats also caused increased pre-implantation loss. Studies in dogs given 0.4 mg/m2 IV (about 0.2 times the oral clinical dose on a mg/m2 basis) of topotecan daily for a month suggest that treatment may cause an increase in the incidence of multinucleated spermatogonial giant cells in the testes. Topotecan may impair fertility in women and men.
Clinical Studies
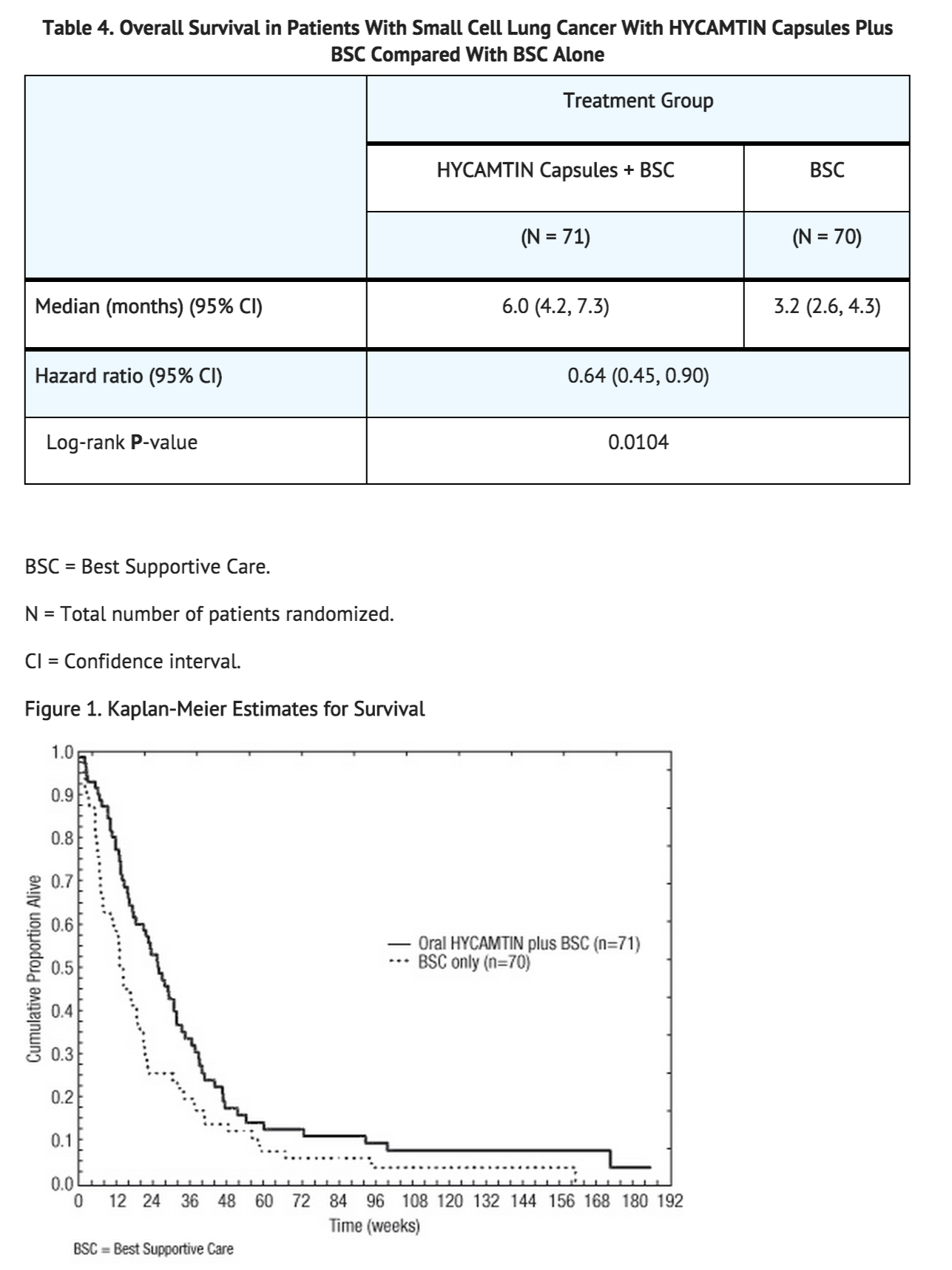
14.1 Small Cell Lung Cancer The efficacy of HYCAMTIN capsules was studied in 141 patients with relapsed SCLC in a randomized, controlled, open-label trial. The patients were prior responders (complete or partial) to first-line chemotherapy, were not considered candidates for standard intravenous chemotherapy, and had relapsed at least 45 days from the end of first-line chemotherapy. Seventy-one patients were randomized to HYCAMTIN capsules (2.3 mg/m2/day administered for 5 consecutive days repeated every 21 days) and Best Supportive Care (BSC) and 70 patients were randomized to BSC alone. The primary objective was to compare the overall survival between the treatment arms. Patients in the arm receiving HYCAMTIN capsules plus BSC received a median of 4 courses (range: 1 to 10) and maintained a median dose intensity of 3.77 mg/m2/week. The median patient age in the arm receiving HYCAMTIN capsules plus BSC and the BSC-alone treatment arm was 60 years and 58 years while the percentage of patients aged >65 years was 34% and 29%, respectively. The majority of patients were Caucasian (99.3%) and male (73%). Eighty percent of patients receiving HYCAMTIN capsules plus BSC previously received carboplatin or cisplatin, and 77% of patients in the BSC-alone arm received prior carboplatin or cisplatin. The arm receiving HYCAMTIN capsules plus BSC included 68% of patients with extensive disease and 28% with liver metastasis. In the BSC- alone arm, 61% of patients had extensive disease and 20% had liver metastases. Both treatment arms recruited 73% males. In the arm receiving HYCAMTIN capsules plus BSC, 18% of patients had prior carboplatin and 62% had prior cisplatin. In the BSC-alone arm, 26% of patients had prior carboplatin and 51% had prior cisplatin.
The arm receiving HYCAMTIN capsules plus BSC showed a statistically significant improvement in overall survival compared with the BSC-alone arm (Log-rank P = 0.0104). Survival results are shown in Table 3 and Figure 1.
How Supplied
The 0.25-mg HYCAMTIN capsules are opaque white to yellowish-white imprinted with HYCAMTIN and 0.25 mg and are available in bottles of 10: NDC 0007-4205-11.
The 1-mg HYCAMTIN capsules are opaque pink imprinted with HYCAMTIN and 1 mg and are available in bottles of 10: NDC 0007-4207-11.
Storage
Store refrigerated 2°C to 8°C (36°F to 46°F). Store the bottles protected from light in the original outer cartons.
HYCAMTIN is a cytotoxic drug. Follow applicable special handling and disposable procedures.
Images
Drug Images
{{#ask: Page Name::Topotecan (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Topotecan (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Bone Marrow Suppression Inform patients that HYCAMTIN decreases blood cell counts such as white blood cells, platelets, and red blood cells. Instruct patients to notify their healthcare provider promptly for fever or other signs of infection such as chills, cough, or burning pain on urination. Advise patients that frequent blood tests will be performed while taking HYCAMTIN to monitor for bone marrow suppression [see Warnings and Precautions (5.1)].
Embryofetal Toxicity Advise patients on pregnancy planning and prevention. Advise females of reproductive potential to use highly effective contraception during treatment and for 1 month following treatment with HYCAMTIN [see Warnings and Precautions (5.6), Use in Specific Populations (8.1, 8.7)].
Advise males with a female sexual partner of reproductive potential to use effective contraception during and for 3 months after treatment [see Nonclinical Toxicology (13.1)].
Nursing Mothers Advise patients to discontinue nursing during treatment with HYCAMTIN [see Use in Specific Populations 8.1, 8.7)].
Infertility Advise male and female patients of the potential risk for impaired fertility and possible family planning options.
Diarrhea Inform patients that HYCAMTIN capsules cause diarrhea which may be severe and life-threatening. Instruct patients how to manage and/or prevent diarrhea and to inform their physician if severe diarrhea occurs during treatment with HYCAMTIN capsules [see Warnings and Precautions (5.2)].
Precautions with Alcohol
Alcohol-Topotecan (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Topotecan (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Topotecan (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.