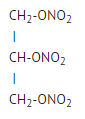Nitroglycerin (Topical ointment)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Nitroglycerin (Topical ointment) is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Nitroglycerin (Topical ointment) FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Topical ointment) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Topical ointment) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Nitroglycerin (Topical ointment) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Topical ointment) in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Topical ointment) in pediatric patients.
Contraindications
- Condition1
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
- Adverse reactions to nitroglycerin are generally dose-related, and almost all of these reactions are the result of nitroglycerin's activity as a vasodilator. Headache, which may be severe, is the most commonly reported side effect. Headache may be recurrent with each, daily dose, especially at higher doses. Transient episodes of lightheadedness, occasionally related to blood pressure changes, may also occur. Hypotension occurs infrequently, but in some patients it may be severe enough to warrant discontinuation of therapy. Syncope, crescendo angina, and rebound hypertension have been reported but are uncommon.
- Allergic reactions to nitroglycerin are also uncommon, and the great majority of those reported have been cases of contact dermatitis or fixed drug eruptions in patients receiving nitroglycerin in ointments or patches. There have been a few reports of genuine anaphylactoid reactions, and these reactions can probably occur in patients receiving nitroglycerin by any route.
- Extremely rarely, ordinary doses of organic nitrates have caused methemoglobinemia in normal-seeming patients; for further discussion of its diagnosis and treatment, see OVERDOSAGE.
- Data are not available to allow estimation of the frequency of adverse reactions during treatment with Nitroglycerin Extended-Release Capsules.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nitroglycerin (Topical ointment) in the drug label.
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitroglycerin (Topical ointment) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nitroglycerin (Topical ointment) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nitroglycerin (Topical ointment) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Nitroglycerin (Topical ointment) in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Nitroglycerin (Topical ointment) in the drug label.
Overdosage
Hemodynamic Effects: The ill effects of nitroglycerin overdose are generally the result of nitroglycerin's capacity to induce vasodilation, venous pooling, reduced cardiac output, and hypotension, These hemodynamic changes may have protean manifestations, including increased intracranial pressure, with any or all of persistent throbbing headache, confusion, and moderate fever; vertigo; palpitations; visual disturbances; nausea and vomiting (possibly with colic and even bloody diarrhea); syncope (especially in the upright posture); air hunger and dyspnea, later followed by reduced ventilatory effort; diaphoresis, with the skin either flushed or cold and clammy; heart block and bradycardia; paralysis; coma; seizures; and death.
Laboratory determinations of serum levels of nitroglycerin and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of nitroglycerin overdose.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of nitroglycerin and its active metabolites. Similarly, it is not known which – if any – of these substances can usefully be removed from the body by hemodialysis.
No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been the subject of controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patients legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
The use of epinephrine or other arterial vasoconstrictors in this setting is likely to do more harm than good.
In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of nitroglycerin overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia: Nitrate ions liberated during metabolism of nitroglycerin can oxidize hemoglobin into methemoglobin. Even in patients totally without cytochrome b5 reductase activity; however, and even assuming that the nitrate moieties of nitroglycerin are quantitatively applied to oxidation of hemoglobin, about 1 mg/kg of nitroglycerin should be required before any of these patients manifests clinically significant (≥10%) methemoglobinemia. In patients with normal reductase function, significant production of methemoglobin should require even larger doses of nitroglycerin. In one study in which 36 patients received 2 to 4 weeks of continuous nitroglycerin therapy at 3.1 to 4.4 mg/hr, the average methemoglobin level measured 0.2%; this was comparable to that observed in parallel patients who received placebo.
Notwithstanding these observations, there are case reports of significant methemoglobinemia in association with moderate overdoses of organic nitrates. None of the affected patients had been thought to be unusually susceptible. Methemoglobin levels are available from most clinical laboratories. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate cardiac output and adequate arterial p02. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
When methemoglobinemia is diagnosed, the treatment of choice is methylene blue, 1 to 2 mg/kg intravenously.
Pharmacology
There is limited information regarding Nitroglycerin (Topical ointment) Pharmacology in the drug label.
Mechanism of Action
- The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle and consequent dilation of peripheral arteries and veins, especially the latter. Dilatation of the veins promotes peripheral pooling of blood and decreases venous return to the heart, thereby reducing left ventricular end-diastolic pressure and pulmonary capillary wedge pressure (preload). Arteriolar relaxation reduces systemic vascular resistance, systolic arterial pressure, and mean arterial pressure (afterload). Dilatation of the coronary arteries also occurs. The relative importance of preload reduction, afterload reduction, and coronary dilatation remains undefined. Dosing regimens for most chronically used drugs are designed to provide plasma concentrations that are continuously greater than a minimally effective concentration. This strategy is inappropriate for organic nitrates. Several well-controlled clinical trials have used exercise testing to assess the anti-anginal efficacy of continuously-delivered nitrates. In the large majority of these trials, active agents were indistinguishable from placebo after 24 hours (or less) of continuous therapy. Attempts to overcome nitrate tolerance by dose escalation, even to doses far in excess of those used acutely, have consistently failed. Only after nitrates had been absent from the body for several hours was their anti-anginal efficacy restored.
Structure
- Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:
- The organic nitrates are vasodilators, active on both arteries and veins. Each Extended-Release Capsule, for oral administration contains 2.5 mg, 6.5 mg, or 9 mg of Nitroglycerin.
- The inactive ingredients in each capsule are corn starch, ethylcellulose, gelatin, lactose monohydrate, pharmaceutical glaze, sugar, talc, and wax. Additionally the 2.5 mg capsule contains FD&C Blue #1, D&C Yellow #10, FD&C Red #40, D&C Red #28; the 6.5 mg capsule contains D&C Yellow #10, FD&C Yellow #6, FD&C Blue #1, D&C Red #33; the 9 mg capsule contains D&C Yellow #10, FD&C Yellow #6, FD&C Green #3, and titanium dioxide.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nitroglycerin (Topical ointment) in the drug label.
Pharmacokinetics
- The volume of distribution of nitroglycerin is about 3 L/kg, and nitroglycerin is cleared from this volume at extremely rapid rates, with a resulting serum half-life of about 3 minutes. *The observed clearance rates (close to 1 L/kg/min) greatly exceed hepatic blood flow; known sites of extrahepatic metabolism include red blood cells and vascular walls.
- The first products in the metabolism of nitroglycerin are inorganic nitrate, and the 1,2- and 1,3-dinitroglycerols. The dinitrates are less effective vasodilators than nitroglycerin, but they are longer-lived in the serum, and their net contribution to the overall effect of chronic nitroglycerin regimens is not known. The dinitrates are further metabolized to (non-vasoactive) mononitrates and, ultimately, to glycerol and carbon dioxide.
- To avoid development of tolerance to nitroglycerin, drug-free intervals of 10-12 hours are known to be sufficient; shorter intervals have not been well studied. In one well-controlled clinical trial, subjects receiving nitroglycerin appeared to exhibit a rebound or withdrawal effect, so that their exercise tolerance at the end of the daily drug-free interval was less than that exhibited by the parallel group receiving placebo.
- Reliable assay techniques for plasma nitroglycerin levels have only recently become available, and studies using these techniques to define the pharmacokinetics of oral nitroglycerin preparations have not been reported. Published studies using older techniques provide results that often differ, in similar experimental settings, by an order of magnitude.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Nitroglycerin (Topical ointment) in the drug label.
Clinical Studies
- Controlled trials of single oral doses of nitroglycerin have demonstrated that nitroglycerin capsules can effectively reduce exercise-related angina for up to 5 hours. Anti-anginal activity is present about 1 hour after ingestion of a capsule.
- Controlled trials of multiple-dose oral nitroglycerin have shown statistically significant anti-anginal efficacy 2½ and 4 hours after a dose when oral nitroglycerin had been administered four times a day for 2 weeks or three times a day for 1 week. As noted above, careful studies with other formulations of nitroglycerin have shown that maintenance of continuous 24-hour plasma levels of nitroglycerin results in insurmountable tolerance. Presumably, the studied 1-week and 2-week regimens of oral nitroglycerin therapy achieved adequate nitrate-free intervals by non-uniformity of dosing interval, with longer intervals overnight. The investigators did not report how subjects interpreted their dosing instructions, and they similarly did not report which dose of the day was the one after which they obtained the end-of-trial exercise results.
- Thus, these studies of oral nitroglycerin should not be interpreted as demonstrations that these regimens provide round-the-clock anti-anginal protection. From large, well-controlled studies of other nitroglycerin formulations, it is reasonable to believe that the maximal achievable daily duration of anti-anginal effect from Nitroglycerin Extended-Release Capsules is about 12 hours.
- In some controlled trials of other organic nitrate formulations, efficacy has declined with time. Because the controlled, multiple-dose trials of oral nitroglycerin did not include exercise tests before the last day of treatment, it is not known how the efficacy of Nitroglycerin Extended-Release Capsules may vary during extended therapy.
How Supplied
Storage
There is limited information regarding Nitroglycerin (Topical ointment) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nitroglycerin (Topical ointment) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nitroglycerin (Topical ointment) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Nitroglycerin (Topical ointment) in the drug label.
Precautions with Alcohol
- Alcohol-Nitroglycerin (Topical ointment) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Nitroglycerin (Topical ointment) |Label Name=Nitroglycerin (Topical ointment)11.png
}}
{{#subobject:
|Label Page=Nitroglycerin (Topical ointment) |Label Name=Nitroglycerin (Topical ointment)11.png
}}