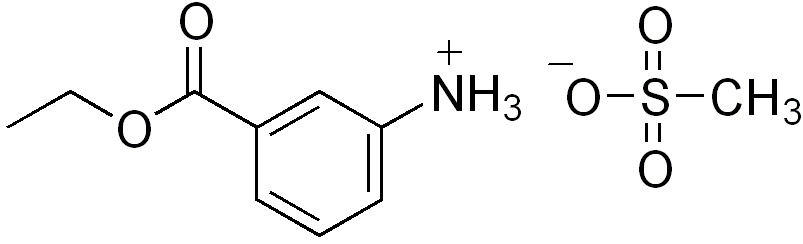
Tricaine mesilate
 | |
| Clinical data | |
|---|---|
| Synonyms | Metacaine Tricaine MS-222 Finquel TMS |
| ATCvet code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H15NO5S |
| Molar mass | 261.296 g/mol |
| 3D model (JSmol) | |
| Melting point | 149.5 °C (301.1 °F) |
| |
| |
| | |
Tricaine mesylate (Tricaine methanesulfonate, TMS, MS-222), is white powder used for anesthesia, sedation, or euthanasia of fish. TMS is the only anesthetic licensed in the United States for fin fish that are intended for human consumption. The drug can have selective toxicity for poikilotherms due to their lower rate of metabolism in the liver.[1]
TMS is a muscle relaxant that operates by preventing action potentials.[citation needed]By blocking action potentials, no signals can be exchanged between the brain and the extremities. There will be no sensory input or muscle contractions which would have been caused by action potential, which includes most muscles.
The optimum concentration may vary with the size and species of the fish, and other variables.
It is easily soluble in water (both fresh and salt) but it drastically decreases the pH of water, increasing the acidity, which may be toxic for fish. Sodium bicarbonate can be used to buffer the solution to a pH range of 6.5-7.5. Usually an equal amount of buffer is added to attain a neutral pH.[2] In salt/marine/sea water, the buffer use may not be necessary because sea water itself has buffering capacity.
The solution of TMS needs to be prepared freshly each time because TMS is light-sensitive and might form toxic by-products upon exposure to light.
References
- ↑ Wayson KA(1976)."Studies on the comparative pharmacology and selective toxicity of tricaine methanesulfonate: metabolism as a basis of the selectivity toxicity in poikilotherms."J Pharmacol Exp Ther198(3):695-708. [PMID 185356]
- ↑ http://www.research.cornell.edu/care/documents/ACUPs/ACUP306.pdf
- Pages with script errors
- Articles with changed ChemSpider identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without UNII source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from February 2013
- Articles with invalid date parameter in template
- General anesthetics
- Benzoates