Simeprevir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Simeprevir is a protease inhibitor that is FDA approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection as a component of a combination antiviral treatment regimen.. Common adverse reactions include pruritus, rash, nausea, hyperbilirubinemia, headache, insomnia and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
OLYSIO Combination Treatment
Administer OLYSIO in combination with other antiviral drugs for the treatment of CHC infection. For specific dosing recommendations for the antiviral drugs used in combination with OLYSIO, refer to their respective prescribing information. OLYSIO monotherapy is not recommended. Administer OLYSIO in combination with either:
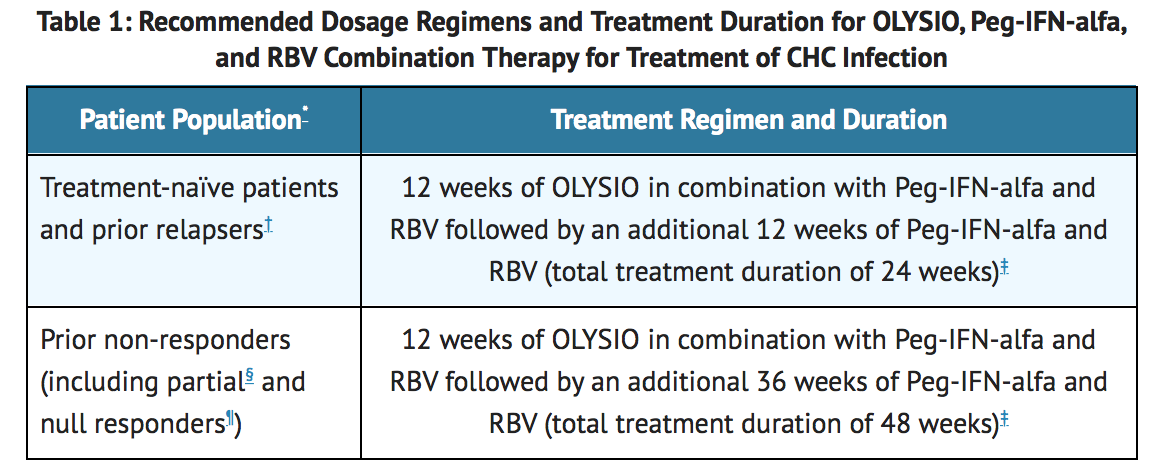
Peg-IFN-alfa and RBV: Table 1 displays the recommended dosage regimen and treatment duration of OLYSIO in combination with Peg-IFN-alfa and RBV. Refer to Table 3 for treatment stopping rules for OLYSIO combination therapy with Peg-IFN-alfa and RBV; or Sofosbuvir: Table 2 displays the recommended dosage regimen and treatment duration of OLYSIO in combination with sofosbuvir. The recommended dosage of OLYSIO is one capsule taken orally once daily with food. The capsule should be swallowed as a whole.
- Includes patients with or without cirrhosis.
† Prior relapser: HCV RNA not detected at the end of prior IFN-based therapy and HCV RNA detected during follow-up. ‡ Recommended duration of treatment if patient does not meet stopping rules (see TABLE 3). § Prior partial responder: prior on-treatment ≥ 2 log10 IU/mL reduction in HCV RNA from baseline at Week 12 and HCV RNA detected at end of prior IFN-based therapy. ¶ Prior null responder: prior on-treatment < 2 log10 reduction in HCV RNA from baseline at Week 12 during prior IFN-based therapy.
Testing Prior to Initiation of OLYSIO in HCV Genotype 1a-Infected Patients
Prior to initiation of treatment with OLYSIO with Peg-IFN-alfa and RBV, screening patients with HCV genotype 1a infection for the presence of virus with the NS3 Q80K polymorphism is strongly recommended and alternative therapy should be considered for patients infected with HCV genotype 1a containing the Q80K polymorphism. Prior to initiation of treatment with OLYSIO with sofosbuvir, screening patients infected with HCV genotype 1a for the presence of virus with the NS3 Q80K polymorphism is not strongly recommended but may be considered. [See INDICATIONS AND USAGE (1)].
Discontinuation of Dosing
Use with Peg-IFN-Alfa and RBV
During treatment, HCV RNA levels should be monitored as clinically indicated using a sensitive assay with a lower limit of quantification of at least 25 IU/mL.
Because patients with an inadequate on-treatment virologic response (i.e., HCV RNA ≥ 25 IU/mL) are not likely to achieve a sustained virologic response (SVR), discontinuation of treatment is recommended in these patients. Table 3 presents treatment stopping rules for patients who experience an inadequate on-treatment virology response at Weeks 4, 12, and 24.
Dosage Adjustment or Interruption
To prevent treatment failure, avoid reducing the dosage of OLYSIO or interrupting treatment. If treatment with OLYSIO is discontinued because of adverse reactions or inadequate on-treatment virologic response, OLYSIO treatment must not be reinitiated.
If adverse reactions potentially related to the antiviral drug(s) used in combination with OLYSIO occur, refer to the instructions outlined in their respective prescribing information for recommendations on dosage adjustment or interruption.
If any of the other antiviral drugs used in combination with OLYSIO for the treatment of CHC infection are permanently discontinued for any reason, OLYSIO should also be discontinued.
Hepatic Impairment
No dosage recommendation can be made for patients with moderate hepatic impairment (Child-Pugh Class B) due to modest increases in simeprevir exposures. OLYSIO is not recommended for patients with severe hepatic impairment (Child-Pugh Class C) due to substantially higher simeprevir exposures. In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including rash and photosensitivity.
The safety and efficacy of OLYSIO have not been studied in HCV-infected patients with moderate or severe hepatic impairment (Child-Pugh Class B or C). Do not administer OLYSIO in combination with Peg-IFN-alfa and RBV in patients with decompensated cirrhosis (moderate or severe hepatic impairment) [see Peg-IFN-alfa prescribing information]. The potential risks and benefits of OLYSIO should be carefully considered prior to use in patients with moderate hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Simeprevir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Simeprevir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Simeprevir FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Simeprevir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Simeprevir in pediatric patients.
Contraindications
There are no specific contraindications to simeprevir. However, as simeprevir should always be administered in combination with other antiviral drugs for the treatment of chronic hepatitis C infection, prescribers should consult the complete prescribing information for these drugs for a description of contraindications.
Warnings
Risk of Serious Adverse Reactions Associated With Combination Treatment. simeprevir should be used in combination with other antiviral drugs for the treatment of CHC infection. Therefore, consult the prescribing information for these drugs before starting therapy with OLYSIO. Warnings and Precautions related to these drugs also apply to their use in simeprevir combination treatment.
Photosensitivity
Photosensitivity reactions have been observed with simeprevir combination therapy. Serious photosensitivity reactions resulting in hospitalization have been observed with simeprevir in combination with Peg-IFN-alfa and ribavirin. Photosensitivity reactions occurred most frequently in the first 4 weeks of treatment, but can occur at any time during treatment. Photosensitivity may present as an exaggerated sunburn reaction, usually affecting areas exposed to light (typically the face, "V" area of the neck, extensor surfaces of the forearms, and dorsa of the hands). Manifestations may include burning, erythema, exudation, blistering, and edema.
Use sun protective measures and limit sun exposure during treatment with OLYSIO. Avoid use of tanning devices during treatment with OLYSIO. Discontinuation of OLYSIO should be considered if a photosensitivity reaction occurs and patients should be monitored until the reaction has resolved. If a decision is made to continue OLYSIO in the setting of a photosensitivity reaction, expert consultation is advised.
Rash
Rash has been observed with OLYSIO combination therapy. Rash occurred most frequently in the first 4 weeks of treatment, but can occur at any time during treatment. Severe rash and rash requiring discontinuation of OLYSIO have been reported in subjects receiving OLYSIO in combination with Peg-IFN-alfa and RBV. Most of the rash events in OLYSIO-treated patients were of mild or moderate severity. Patients with mild to moderate rashes should be followed for possible progression of rash, including the development of mucosal signs (e.g., oral lesions, conjunctivitis) or systemic symptoms. If the rash becomes severe, OLYSIO should be discontinued. Patients should be monitored until the rash has resolved.
Sulfa Allergy
OLYSIO contains a sulfonamide moiety. In subjects with a history of sulfa allergy (n=16), no increased incidence of rash or photosensitivity reactions has been observed. However, there are insufficient data to exclude an association between sulfa allergy and the frequency or severity of adverse reactions observed with the use of OLYSIO.
Risk of Adverse Reactions or Reduced Therapeutic Effect Due to Drug Interactions
Co-administration of OLYSIO with substances that are moderate or strong inducers or inhibitors of cytochrome P450 3A (CYP3A) is not recommended as this may lead to significantly lower or higher exposure of simeprevir, respectively, which may result in reduced therapeutic effect or adverse reactions
Adverse Reactions
Clinical Trials Experience
OLYSIO should be administered in combination with other antiviral drugs. Refer to the prescribing information of the antiviral drugs used in combination with OLYSIO for a description of adverse reactions associated with their use.
The following serious and otherwise important adverse drug reactions (ADRs) are discussed in detail in another section of the labeling:
- Photosensitivity
- Rash
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions when Used in Combination with Peg-IFN-Alfa and RBV
The safety profile of OLYSIO in combination with Peg-IFN-alfa and RBV in patients with HCV genotype 1 infection who were treatment-naïve or who had previously relapsed following interferon therapy with or without RBV is based on pooled data from three Phase 3 trials [see CLINICAL STUDIES (14)]. These trials included a total of 1178 subjects who received OLYSIO or placebo in combination with 24 or 48 weeks of Peg-IFN-alfa and RBV. Of the 1178 subjects, 781 subjects were randomized to receive OLYSIO 150 mg once daily for 12 weeks and 397 subjects were randomized to receive placebo once daily for 12 weeks.
In the pooled Phase 3 safety data, the majority of the adverse reactions reported during 12 weeks treatment with OLYSIO in combination with Peg-IFN-alfa and RBV were Grade 1 to 2 in severity. Grade 3 or 4 adverse reactions were reported in 23% of subjects receiving OLYSIO in combination with Peg-IFN-alfa and RBV versus 25% of subjects receiving placebo in combination with Peg-IFN-alfa and RBV. Serious adverse reactions were reported in 2% of subjects receiving OLYSIO in combination with Peg-IFN-alfa and RBV and in 3% of subjects receiving placebo in combination with Peg-IFN-alfa and RBV. Discontinuation of OLYSIO or placebo due to adverse reactions occurred in 2% and 1% of subjects receiving OLYSIO with Peg-IFN-alfa and RBV and subjects receiving placebo with Peg-IFN-alfa and RBV, respectively.
The following table lists adverse reactions (all Grades) that occurred with at least 3% higher frequency among subjects receiving OLYSIO 150 mg once daily in combination with Peg-IFN-alfa and RBV, compared to subjects receiving placebo in combination with Peg-IFN-alfa and RBV, during the first 12 weeks of treatment in the pooled Phase 3 trials in subjects who were treatment-naïve or who had previously relapsed after Peg-IFN-alfa and RBV therapy (see TABLE 4).
Rash and Photosensitivity
In the Phase 3 clinical trials, rash (including photosensitivity reactions) was observed in 28% of OLYSIO-treated subjects compared to 20% of placebo-treated subjects during the 12 weeks of treatment with OLYSIO or placebo in combination with Peg-IFN-alfa and RBV. Fifty-six percent (56%) of rash events in the OLYSIO group occurred in the first 4 weeks, with 42% of cases occurring in the first 2 weeks. Most of the rash events in OLYSIO-treated subjects were of mild or moderate severity (Grade 1 or Grade 2). Severe (Grade 3) rash occurred in 1% of OLYSIO-treated subjects and in none of the placebo-treated subjects. There were no reports of life-threatening (Grade 4) rash. Discontinuation of OLYSIO or placebo due to rash occurred in 1% of OLYSIO-treated subjects, compared to less than 1% of placebo-treated subjects. The frequencies of rash and photosensitivity reactions were higher in subjects with higher simeprevir exposures.
All subjects enrolled in the Phase 3 trials were directed to use sun protection measures. In these trials, adverse reactions under the specific category of photosensitivity were reported in 5% of OLYSIO-treated subjects compared to 1% of placebo-treated subjects during the 12 weeks of treatment with OLYSIO or placebo in combination with Peg-IFN-alfa and RBV. Most photosensitivity reactions in OLYSIO-treated subjects were of mild or moderate severity (Grade 1 or 2). Two OLYSIO-treated subjects experienced photosensitivity reactions which resulted in hospitalization. No life-threatening photosensitivity reactions were reported.
Dyspnea
During the 12 weeks of treatment with OLYSIO, dyspnea was reported in 12% of OLYSIO-treated subjects compared to 8% of placebo-treated subjects (all grades; pooled Phase 3 trials). All dyspnea events reported in OLYSIO-treated subjects were of mild or moderate severity (Grade 1 or 2). There were no Grade 3 or 4 dyspnea events reported and no subjects discontinued treatment with OLYSIO due to dyspnea. Sixty-one percent (61%) of dyspnea events occurred in the first 4 weeks of treatment with OLYSIO.
Laboratory abnormalities
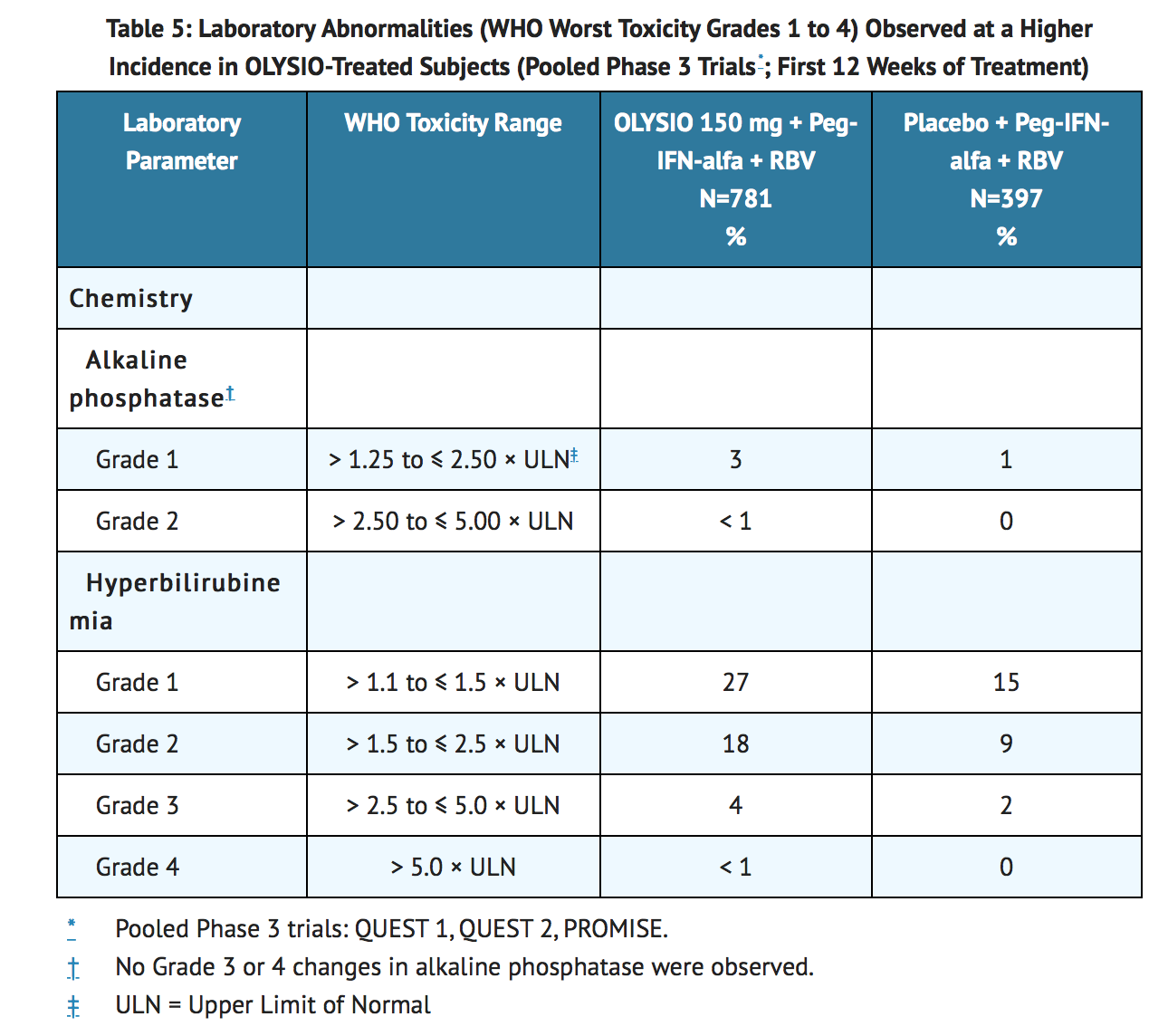
There were no differences between treatment groups for the following laboratory parameters: hemoglobin, neutrophils, platelets, aspartate aminotransferase, alanine aminotransferase, amylase, or serum creatinine. Laboratory abnormalities that were observed at a higher incidence in OLYSIO-treated subjects than in placebo-treated subjects are listed in Table 5.
Elevations in bilirubin were predominately mild to moderate (Grade 1 or 2) in severity, and included elevation of both direct and indirect bilirubin. Elevations in bilirubin occurred early after treatment initiation, peaking by study Week 2, and were rapidly reversible upon cessation of OLYSIO. Bilirubin elevations were generally not associated with elevations in liver transaminases.
Adverse Reactions when Used with Sofosbuvir
In the COSMOS trial, the most common (> 10%) adverse reactions reported during 12 weeks treatment with OLYSIO in combination with sofosbuvir without RBV were fatigue (25%), headache (21%), nausea (21%), insomnia (14%) and pruritus (11%). Rash and photosensitivity were reported in 11% and 7% of subjects, respectively. During 24 weeks treatment with OLYSIO in combination with sofosbuvir, dizziness (16%), and diarrhea (16%) were also commonly reported.
Postmarketing Experience
There is limited information regarding Simeprevir Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Simeprevir Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy
OLYSIO must be administered in combination with other antiviral drugs. Refer to prescribing information of the drugs used in combination with OLYSIO for information regarding use in pregnancy.
Pregnancy Category C
Risk Summary
Adequate and well-controlled trials with OLYSIO have not been conducted in pregnant women. In animal reproduction studies with simeprevir, embryofetal developmental toxicity was observed at drug exposures higher than human exposure at the recommended clinical dose. OLYSIO should be used during pregnancy only if the potential benefit justifies the potential risk. Female patients of childbearing potential should use an effective contraceptive method.
If OLYSIO is administered with Peg-IFN-alfa and RBV, refer to the prescribing information for Peg-IFN-alfa and RBV for information on use in pregnancy.
Animal Data
Simeprevir showed no teratogenicity in rats and mice at exposures 0.5 times (in rats) and 6 times (in mice) the mean area under the plasma concentration time curve (AUC) in humans at the recommended dose of 150 mg once daily.
In a mouse embryofetal study at doses up to 1000 mg/kg, simeprevir resulted in early and late in utero fetal losses and early maternal deaths at an exposure approximately 6 times higher than the mean AUC in humans at the recommended 150 mg daily dose. Significantly decreased fetal weights and an increase in fetal skeletal variations were seen at exposures approximately 4 times higher than the mean AUC in humans at the recommended daily dose.
In a rat pre- and postnatal study, maternal animals were exposed to simeprevir during gestation and lactation at doses up to 1000 mg/kg/day. In pregnant rats, simeprevir resulted in early deaths at 1000 mg/kg/day corresponding to exposures similar to the mean AUC in humans at the recommended 150 mg once daily dose. Significant reduction in body weight gain was seen at an exposure 0.7 times the mean AUC in humans at the recommended 150 mg once daily dose. The developing rat offspring exhibited significantly decreased body weight and negative effects on physical growth (delay and small size) and development (decreased motor activity) following simeprevir exposure in utero (via maternal dosing) and during lactation (via maternal milk to nursing pups) at a maternal exposure similar to the mean AUC in humans at the recommended 150 mg once daily dose. Subsequent survival, behavior and reproductive capacity were not affected.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Simeprevir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Simeprevir during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Simeprevir in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Simeprevir in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Simeprevir in geriatric settings.
Gender
There is no FDA guidance on the use of Simeprevir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Simeprevir with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Simeprevir in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Simeprevir in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Simeprevir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Simeprevir in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Simeprevir Administration in the drug label.
Monitoring
There is limited information regarding Simeprevir Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Simeprevir and IV administrations.
Overdosage
There is limited information regarding Simeprevir overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Simeprevir Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Simeprevir Mechanism of Action in the drug label.
Structure
There is limited information regarding Simeprevir Structure in the drug label.
Pharmacodynamics
There is limited information regarding Simeprevir Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Simeprevir Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Simeprevir Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Simeprevir Clinical Studies in the drug label.
How Supplied
There is limited information regarding Simeprevir How Supplied in the drug label.
Storage
There is limited information regarding Simeprevir Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Simeprevir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Simeprevir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Simeprevir Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Simeprevir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Simeprevir Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Simeprevir Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.