Ofloxacin (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
*Fluoroquinolones, including ofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
|
Overview
Ofloxacin (oral) is an anti-bacterial agent that is FDA approved for the treatment of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include pruritus, diarrhea, nausea, vomiting, dizziness, headache, insomnia, burning sensation in eye, pain in eye, pruritus of genital organs(female), vaginitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute bacterial exacerbation of chronic bronchitis
- Dosing Information
- 400 mg ORALLY every 12 hours for 10 days
Bacterial conjunctivitis
- Dosing Information
- 0.3% ophthalmic solution, instill 1 or 2 drops in affected eye(s) every 2 to 4 hours for 2 days, then 1 to 2 drops 4 times daily on days 3 through 7
Chlamydial infection
- Dosing Information
- 300 mg ORALLY every 12 hours daily for 7 days
Chronic purulent otitis media - Perforation of tympanic membrane
- Dosing Information
- Otic solution: 10 drops into affected ear(s) twice daily for 14 days
Community acquired pneumonia
- Dosing Information
- 400 mg ORALLY every 12 hours for 10 days
Corneal ulcer
- Dosing Information
- (0.3% ophthalmic solution) days 1 and 2, instill 1 to 2 drops in affected eye(s) every 30 minutes while awake, and awaken to instill 1 to 2 drops at approximately 4 and 6 hours after going to bed; days 3 to 7, instill 1 to 2 drops hourly while awake; days 7 to 9, through treatment completion, instill 1 to 2 drops 4 times daily
Cystitis, Uncomplicated
- Dosing Information
- (Due to E coli or K pneumoniae) 200 mg ORALLY every 12 hours for 3 days.
- (Due to other susceptible organisms) 200 mg ORALLY every 12 hours for 7 days.
Gonorrhea
- Dosing Information
- Uncomplicated, 400 mg ORALLY as a single dose; fluoroquinolones are no longer recommended by the CDC in the United States for the treatment of gonorrhea.
Infection due to Staphylococcus aureus
Infection of skin AND/OR subcutaneous tissue, Uncomplicated
- Dosing Information
- 400 mg ORALLY every 12 hours for 10 days
Nongonococcal urethritis
- Dosing Information
- 300 mg ORALLY twice daily for 7 days
Otitis externa
- Dosing Information
- (Otic solution) 10 drops into affected ear(s) once daily for 7 days
Pelvic inflammatory disease
- Dosing Information
- 400 mg ORALLY every 12 hours for 10 to 14 days with or without metronidazole 500 mg ORALLY twice daily for 14 days; however, fluoroquinolones are no longer recommended by the CDC in the United States for the treatment of pelvic inflammatory disease.
Prostatitis
- Dosing Information
- 300 mg ORALLY every 12 hours for 6 weeks
Urinary tract infectious disease, Complicated
- Dosing Information
- 200 mg ORALLY every 12 hours for 10 days
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Epididymitis
- Developed by:
- Class of Recommendation: Adult, Class IIb
- Strength of Evidence: Adult, Category C
- Dosing Information
- (Caused by enteric organisms, or with negative gonococcal nucleic acid amplification test) 300 mg ORALLY twice daily for 10 days
Non–Guideline-Supported Use
- Traveler's diarrhea
- Dosing Information
- 300 mg ORALLY twice daily for 1 to 3 days
- Infection of bone - Infectious disorder of joint
- Infective cholangitis
- Leprosy
- Tuberculosis
- Typhoid fever
- Upper respiratory infection
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Acute otitis media - Tympanostomy
- Dosing Information
- (Otic solution) 1 to 12 years of age, 5 drops in affected ear(s) twice daily for 10 days
Bacterial conjunctivitis
- Dosing Information
- (1 year or older) 0.3% ophthalmic solution, instill 1 or 2 drops in affected eye(s) every 2 to 4 hours for 2 days, then 1 to 2 drops 4 times daily on days 3 through 7
Chronic purulent otitis media - Perforation of tympanic membrane
- Dosing Information
- (Otic solution; 12 years and older) 10 drops in affected ear(s) twice daily for 14 days
Corneal ulcer
- Dosing Information
- (0.3% ophthalmic solution; 1 year or older) days 1 and 2, instill 1 to 2 drops in affected eye(s) every 30 minutes while awake, and awaken to instill 1 to 2 drops at approximately 4 and 6 hours after going to bed; days 3 to 7, instill 1 to 2 drops hourly while awake; days 7 to 9, through treatment completion, instill 1 to 2 drops 4 times daily.
Infection due to Staphylococcus aureus (over 1 year old)
Otitis externa
- Dosing Information
- (Otic solution; 6 mo to 13 years of age) 5 drops in affected ear(s) once daily for 7 days
- (Otic solution; 13 years and older) 10 drops into affected ear(s) once daily for 7 days
Off-Label Use and Dosage (Pediatric)
Non–Guideline-Supported Use
- Upper respiratory infection
- Urinary tract infectious disease, Complicated
Contraindications
- Ofloxacin tablets are contraindicated in persons with a history of hypersensitivity associated with the use of ofloxacin or any member of the quinolone group of antimicrobial agents.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
*Fluoroquinolones, including ofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
|
Tendinopathy and Tendon Rupture
- Fluoroquinolones, including ofloxacin, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendons have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. Ofloxacin should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
- THE SAFETY AND EFFICACY OF OFLOXACIN IN PEDIATRIC PATIENTS AND ADOLESCENTS (UNDER THE AGE OF 18 YEARS), PREGNANT WOMEN, AND LACTATING WOMEN HAVE NOT BEEN ESTABLISHED (see PRECAUTIONS, Pediatric Use, Pregnancy, and Nursing MothersSubsections).
- In the immature rat, the oral administration of ofloxacin at 5 to 16 times the recommended maximum human dose based on mg/kg or 1 to 3 times based on mg/m2 increased the incidence and severity of osteochondrosis. The lesions did not regress after 13 weeks of drug withdrawal. Other quinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species (see ANIMAL PHARMACOLOGY).
Exacerbation of Myasthenia Gravis
- Fluoroquinolones, including ofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Postmarketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid ofloxacin in patients with known history of myasthenia gravis (see PRECAUTIONS, Information for Patientsand ADVERSE REACTIONS, Postmarketing Adverse Events).
Central Nervous System Effects:
- Convulsions, increased intracranial pressure, (including pseudotumor cerebri), and toxic psychosis have been reported in patients receiving quinolones, including ofloxacin. Quinolones, including ofloxacin, may also cause central nervous system stimulation which may lead to: tremors, restlessness/agitation, nervousness/anxiety, lightheadedness, confusion, hallucinations, paranoia and depression, nightmares, insomnia, and rarely suicidal thoughts or acts. These reactions may occur following the first dose. If these reactions occur in patients receiving ofloxacin, the drug should be discontinued and appropriate measures instituted. Insomnia may be more common with ofloxacin than some other products in the quinolone class. As with all quinolones, ofloxacin should be used with caution in patients with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction) (see PRECAUTIONS, General, Information for Patients, Drug Interactionsand ADVERSE REACTIONS).
Hypersensitivity Reactions
- Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones, including ofloxacin. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat, or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath, and acute respiratory distress), dyspnea, urticaria, itching, and other serious skin reactions. This drug should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated (see PRECAUTIONS and ADVERSE REACTIONS).
- Other serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including ofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
- The drug should be discontinued immediately at the first appearance of skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted (see PRECAUTIONS, Information for Patients and ADVERSE REACTIONS).
Peripheral Neuropathy
- Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including ofloxacin. Symptoms may occur soon after initiation of ofloxacin and may be irreversible. Ofloxacin should be discontinued immediately if the patient experiences symptoms of neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations in sensations including light touch, pain, temperature, position sense, and vibratory sensation.
- Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ofloxacin tablets, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated (see ADVERSE REACTIONS).
- Ofloxacin has not been shown to be effective in the treatment of syphilis.
- Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with ofloxacin for gonorrhea should have a follow-up serologic test for syphilis after three months and, if positive, treatment with an appropriate antimicrobial should be instituted.
Precautions
General
- Prescribing ofloxacin tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Adequate hydration of patients receiving ofloxacin should be maintained to prevent the formation of a highly concentrated urine.
- Administer ofloxacin with caution in the presence of renal or hepatic insufficiency/impairment. In patients with known or suspected renal or hepatic insufficiency/impairment, careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of ofloxacin may be reduced. In patients with impaired renal function (creatinine clearance ≤ 50 mg/mL), alteration of the dosage regimen is necessary(see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
- Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (e.g., burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, “V” area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of quinolones after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Drug therapy should be discontinued if photosensitivity/phototoxicity occurs (see ADVERSE REACTIONS, Postmarketing Adverse Events).
- As with other quinolones, ofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction) (see WARNINGS and Drug Interactions).
- A possible interaction between oral hypoglycemic drugs (e.g., glyburide/glibenclamide) or with insulin and fluoroquinolone antimicrobial agents have been reported resulting in a potentiation of the hypoglycemic action of these drugs. The mechanism for this interaction is not known. If a hypoglycemic reaction occurs in a patient being treated with ofloxacin, discontinue ofloxacin immediately and consult a physician (see Drug Interactions and ADVERSE REACTIONS).
- As with any potent drug, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy.
Torsade de Pointes
- Some quinolones, including ofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsade de pointes have been spontaneously reported during postmarketing surveillance in patients receiving quinolones, including ofloxacin. Ofloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents.
Adverse Reactions
Clinical Trials Experience
- The following is a compilation of the data for ofloxacin based on clinical experience with both the oral and intravenous formulations. The incidence of drug-related adverse reactions in patients during Phase 2 and 3 clinical trials was 11%. Among patients receiving multiple-dose therapy, 4% discontinued ofloxacin due to adverse experiences.
- In clinical trials, the following events were considered likely to be drug-related in patients receiving multiple doses of ofloxacin:
- nausea 3%, insomnia 3%, headache 1%, dizziness 1%, diarrhea 1%, vomiting 1%, rash 1%, pruritus 1%, external genital pruritus in women 1%, vaginitis 1%, dysgeusia 1%.
- In clinical trials, the most frequently reported adverse events, regardless of relationship to drug, were:
- nausea 10%, headache 9%, insomnia 7%, external genital pruritus in women 6%, dizziness 5%, vaginitis 5%, diarrhea 4%, vomiting 4%.
- In clinical trials, the following events, regardless of relationship to drug, occurred in 1 to 3% of patients:
- abdominal pain and cramps, chest pain, decreased appetite, dry mouth, dysgeusia, fatigue, flatulence, gastrointestinal distress, nervousness, pharyngitis, pruritus, fever, rash, sleep disorders, somnolence, trunk pain, vaginal discharge, visual disturbances, and constipation.
- Additional events, occurring in clinical trials at a rate of less than 1%, regardless of relationship to drug, were:
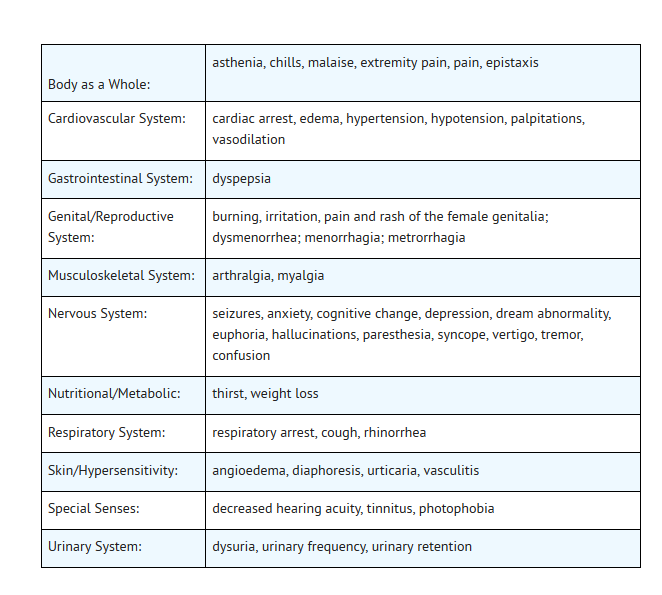
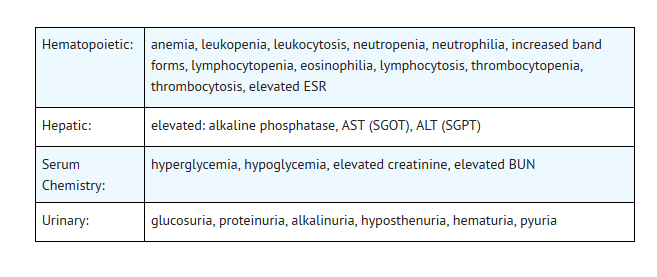
- The following laboratory abnormalities appeared in ≥ 1% of patients receiving multiple doses of ofloxacin. It is not known whether these abnormalities were caused by the drug or the underlying conditions being treated.
Postmarketing Experience
- Additional adverse events, regardless of relationship to drug, reported from worldwide marketing experience with quinolones, including ofloxacin:
Clinical
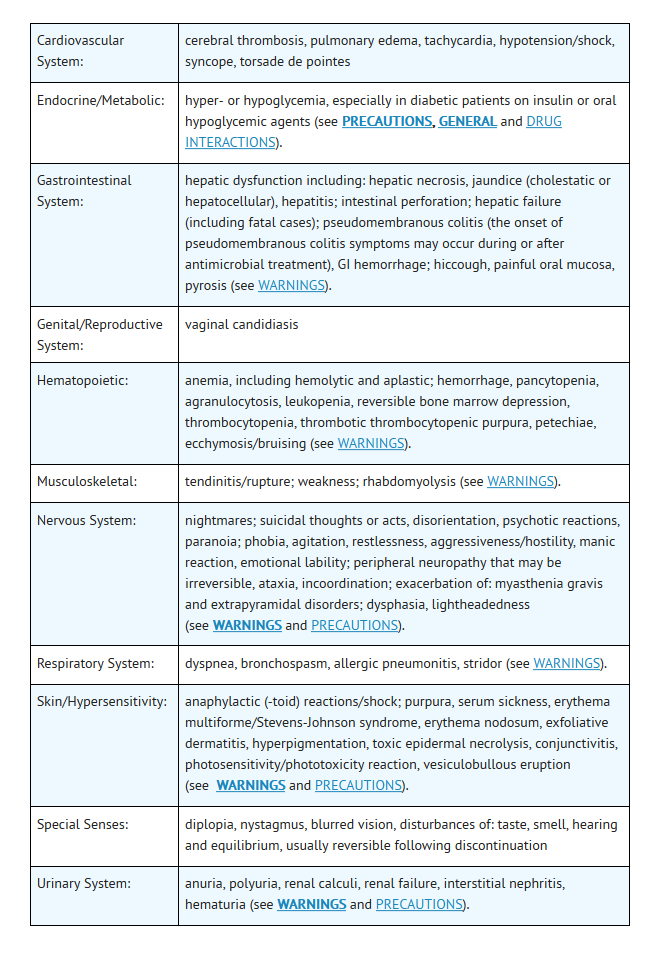
Laboratory
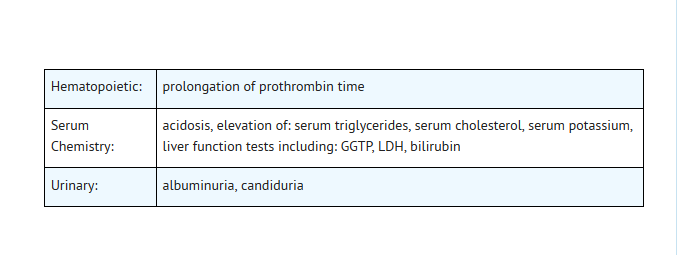
- In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with other quinolones. The relationship of the drugs to these events is not presently established.
- CRYSTALLURIA and CYLINDRURIA HAVE BEEN REPORTED with other quinolones.
Drug Interactions
Antacids, Sucralfate, Metal Cations, Multivitamins
- Quinolones form chelates with alkaline earth and transition metal cations. Administration of quinolones with antacids containing calcium, magnesium, or aluminum, with sucralfate, with divalent or trivalent cations such as iron, or with multivitamins containing zinc or with didanosine, chewable/buffered tablets or the pediatric powder for oral solution may substantially interfere with the absorption of quinolones resulting in systemic levels considerably lower than desired. These agents should not be taken within the two-hour period before or within the two-hour period after ofloxacin administration (see DOSAGE AND ADMINISTRATION).
Caffeine
- Interactions between ofloxacin and caffeine have not been detected.
Cimetidine
- Cimetidine has demonstrated interference with the elimination of some quinolones. This interference has resulted in significant increases in half-life and AUC of some quinolones. The potential for interaction between ofloxacin and cimetidine has not been studied.
Cyclosporine
- Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with some other quinolones. The potential for interaction between ofloxacin and cyclosporine has not been studied.
Drugs Metabolized by Cytochrome P450 Enzymes
- Most quinolone antimicrobial drugs inhibit cytochrome P450 enzyme activity. This may result in a prolonged half-life for some drugs that are also metabolized by this system (e.g., cyclosporine, theophylline/methylxanthines, warfarin) when coadministered with quinolones. The extent of this inhibition varies among different quinolones (see other Drug Interactions).
Non Steroidal Anti-Inflammatory Drugs
- The concomitant administration of a non-steroidal anti-inflammatory drug with a quinolone, including ofloxacin, may increase the risk of CNS stimulation and convulsive seizures (see WARNINGS and PRECAUTIONS , General).
Probenecid
- The concomitant use of probenecid with certain other quinolones has been reported to affect renal tubular secretion. The effect of probenecid on the elimination of ofloxacin has not been studied.
Theophylline
- Steady-state theophylline levels may increase when ofloxacin and theophylline are administered concurrently. As with other quinolones, concomitant administration of ofloxacin may prolong the half-life of theophylline, elevate serum theophylline levels, and increase the risk of theophylline-related adverse reactions. Theophylline levels should be closely monitored and theophylline dosage adjustments made, if appropriate, when ofloxacin is coadministered. Adverse reactions (including seizures) may occur with or without an elevation in the serum theophylline level (see WARNINGS and PRECAUTIONS , General).
Warfarin
- Some quinolones have been reported to enhance the effects of the oral anticoagulant warfarin or its derivatives. Therefore, if a quinolone antimicrobial is administered concomitantly with warfarin or its derivatives, the prothrombin time or other suitable coagulation test should be closely monitored.
Antidiabetic Agents (e.g., Insulin, Glyburide/Glibenclamide)
- Since disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concurrently with quinolones and an antidiabetic agent, careful monitoring of blood glucose is recommended when these agents are used concomitantly (see PRECAUTIONS , General and Information for Patients).
Interaction With Laboratory or Diagnostic Testing
- Some quinolones, including ofloxacin, may produce false-positive urine screening results for opiates using commercially available immunoassay kits. Confirmation of positive opiate screens by more specific methods may be necessary.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Teratogenic Effects
Pregnancy Category C
- Ofloxacin has not been shown to have any teratogenic effects at oral doses as high as 810 mg/kg/day (11 times the recommended maximum human dose based on mg/m2 or 50 times based on mg/kg) and 160 mg/kg/day (4 times the recommended maximum human dose based on mg/m2 or 10 times based on mg/kg) when administered to pregnant rats and rabbits, respectively. Additional studies in rats with oral doses up to 360 mg/kg/day (5 times the recommended maximum human dose based on mg/m2 or 23 times based on mg/kg) demonstrated no adverse effect on late fetal development, labor, delivery, lactation, neonatal viability, or growth of the newborn. Doses equivalent to 50 and 10 times the recommended maximum human dose of ofloxacin (based on mg/kg) were fetotoxic (i.e., decreased fetal body weight and increased fetal mortality) in rats and rabbits, respectively. Minor skeletal variations were reported in rats receiving doses of 810 mg/kg/day, which is more than 10 times higher than the recommended maximum human dose based on mg/m2.
- There are, however, no adequate and well-controlled studies in pregnant women. Ofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (see WARNINGS).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ofloxacin (oral) in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Ofloxacin (oral) during labor and delivery.
Nursing Mothers
- In lactating females, a single oral 200 mg dose of ofloxacin resulted in concentrations of ofloxacin in milk that were similar to those found in plasma. Because of the potential for serious adverse reactions from ofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see WARNINGS and ADVERSE REACTIONS).
Pediatric Use
- Safety and effectiveness in pediatric patients and adolescents below the age of 18 years have not been established. Ofloxacin causes arthropathy (arthrosis) and osteochondrosis in juvenile animals of several species (see WARNINGS).
Geriatic Use
- Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as ofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing ofloxacin to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue ofloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur (see Boxed Warning; WARNINGS; and ADVERSE REACTIONS, Postmarketing Adverse Event Reports).
- In phase 2/3 clinical trials with ofloxacin, 688 patients (14.2%) were ≥ 65 years of age. Of these, 436 patients (9%) were between the ages of 65 and 74 and 252 patients (5.2%) were 75 years or older. There was no apparent difference in the frequency or severity of adverse reactions in elderly adults compared with younger adults. The pharmacokinetic properties of ofloxacin in elderly subjects are similar to those in younger subjects. Drug absorption appears to be unaffected by age. Dosage adjustment is necessary for elderly patients with impaired renal function (creatinine clearance rate ≤ 50 mL/min) due to reduced clearance of ofloxacin. In comparative studies, the frequency and severity of most drug-related nervous system events in patients ≥65 years of age were comparable for ofloxacin and control drugs. The only differences identified were an increase in reports of insomnia (3.9% vs. 1.5%) and headache (4.7% vs. 1.8%) with ofloxacin. It is important to note that these geriatric safety data are extracted from 44 comparative studies where the adverse reaction information from 20 different controls (other antibiotics or placebo) were pooled for comparison with ofloxacin. The clinical significance of such a comparison is not clear(see CLINICAL PHARMACOLOGYand DOSAGE AND ADMINISTRATION).
- Elderly patients may be more sensitive to drug-associated effects on the QT interval. Therefore, precaution should be taken when using ofloxacin with concomitant drugs that can result in prolongation of the QT interval (e.g. Class IA or Class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g. known QT prolongation, uncorrected hypokalemia)(See PRECAUTIONS: General : Torsade de pointes).
Gender
- There is no FDA guidance on the use of Ofloxacin (oral) with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Ofloxacin (oral) with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Ofloxacin (oral) in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Ofloxacin (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Ofloxacin (oral) in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Ofloxacin (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
- The usual dose of ofloxacin tablets is 200 mg to 400 mg orally every 12 h as described in the following dosing chart. These recommendations apply to patients With normal renal function (i.e., creatinine clearance > 50 mL/min). For patients with altered renal function (i.e., creatinine clearance ≤ 50 mL/min), see the Patients With Impaired Renal Function subsection.
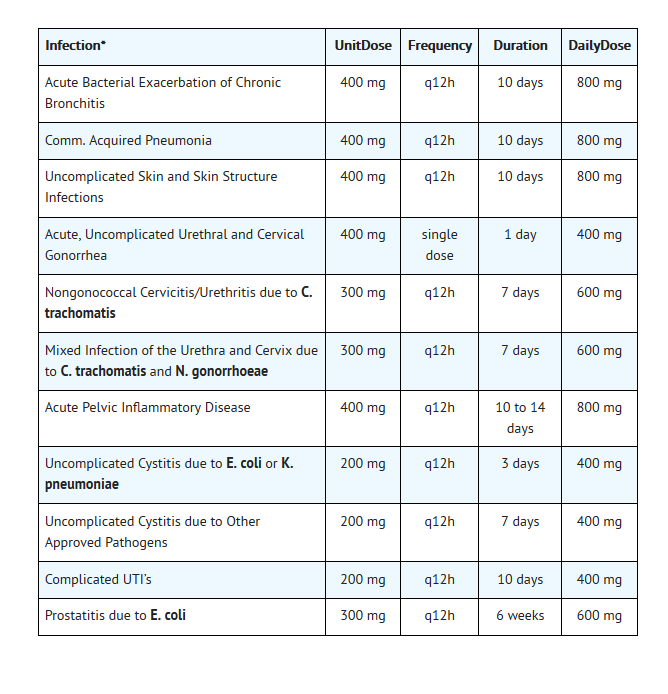
- DUE TO THE DESIGNATED PATHOGENS (see INDICATIONS AND USAGE).
- Antacids containing calcium, magnesium, or aluminum; sucralfate; divalent or trivalent cations such as iron; or multivitamins containing zinc; or didanosine, chewable/buffered tablets or the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking ofloxacin (see PRECAUTIONS).
Patients With Impaired Renal Function
- Dosage should be adjusted for patients with a creatinine clearance ≤ 50 mL/min. After a normal initial dose, dosage should be adjusted as follows:

- When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance.
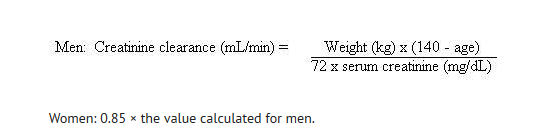
- Women: 0.85 × the value calculated for men.
- The serum creatinine should represent a steady-state of renal function.
Patients With Cirrhosis
- The excretion of ofloxacin may be reduced in patients with severe liver function disorders (e.g., cirrhosis with or without ascites). A maximum dose of 400 mg of ofloxacin per day should therefore not be exceeded.
Monitoring
- Steady-state theophylline levels may increase when ofloxacin and theophylline are administered concurrently. Theophylline levels should be closely monitored and theophylline dosage adjustments made, if appropriate, when ofloxacin is coadministered. Adverse reactions (including seizures) may occur with or without an elevation in the serum theophylline level.
- If a quinolone antimicrobial is administered concomitantly with warfarin or its derivatives, the prothrombin time or other suitable coagulation test should be closely monitored.
- Since disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concurrently with quinolones and an antidiabetic agent, careful monitoring of blood glucose is recommended when these agents are used concomitantly.
IV Compatibility
- There is limited information regarding IV Compatibility of Ofloxacin (oral) in the drug label.
Overdosage
- Information on overdosage with ofloxacin is limited. One incident of accidental overdosage has been reported. In this case, an adult female received 3 grams of ofloxacin intravenously over 45 minutes. A blood sample obtained 15 minutes after the completion of the infusion revealed an ofloxacin level of 39.3 mcg/mL. In 7 h, the level had fallen to 16.2 mcg/mL, and by 24 h to 2.7 mcg/mL. During the infusion, the patient developed drowsiness, nausea, dizziness, hot and cold flushes, subjective facial swelling and numbness, slurring of speech, and mild to moderate disorientation. All complaints except the dizziness subsided within 1 h after discontinuation of the infusion. The dizziness, most bothersome while standing, resolved in approximately 9 h. Laboratory testing reportedly revealed no clinically significant changes in routine parameters in this patient.
- In the event of an acute overdose, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Ofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Pharmacology
Mechanism of Action
There is limited information regarding Ofloxacin (oral) Mechanism of Action in the drug label.
Structure
- Ofloxacin tablets are a synthetic broad-spectrum antimicrobial agent for oral administration. Chemically, ofloxacin, USP, a fluorinated carboxyquinolone, is the racemate, (±)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. The chemical structure is:
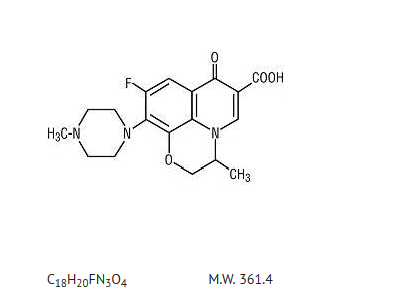
- Ofloxacin, USP is an off-white to pale yellow crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine. The relative solubility characteristics of ofloxacin USP at room temperature, as defined by USP nomenclature, indicate that ofloxacin, USP is considered to be soluble in aqueous solutions with pH between 2 and 5. It is sparingly to slightly soluble in aqueous solutions with pH 7 (solubility falls to 4 mg/mL) and freely soluble in aqueous solutions with pH above 9. Ofloxacin USP has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Fe+3> Al+3> Cu +2> Ni +2> Pb+2> Zn+2> Mg+2> Ca +2> Ba +2
- Ofloxacin tablets, USP contain the following inactive ingredients: lactose monohydrate, pregelatinized maize starch, hydroxy propyl methyl cellulose, talc, magnesium stearate, polyethylene glycol, sodium starch glycolate, and titanium dioxide. Additionally, the 200 mg and 400 mg tablets contain iron oxide yellow.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Ofloxacin (oral) in the drug label.
Pharmacokinetics
- Following oral administration, the bioavailability of ofloxacin in the tablet formulation is approximately 98%. Maximum serum concentrations are achieved one to two hours after an oral dose. Absorption of ofloxacin after single or multiple doses of 200 to 400 mg is predictable, and the amount of drug absorbed increases proportionately with the dose. Ofloxacin has biphasic elimination. Following multiple oral doses at steady-state administration, the half-lives are approximately 4 to 5 hours and 20 to 25 hours. However, the longer half-life represents less than 5% of the total AUC. Accumulation at steady-state can be estimated using a half-life of 9 hours. The total clearance and volume of distribution are approximately similar after single or multiple doses. Elimination is mainly by renal excretion. The following are mean peak serum concentrations in healthy 70 to 80 kg male volunteers after single oral doses of 200, 300, or 400 mg of ofloxacin or after multiple oral doses of 400 mg.
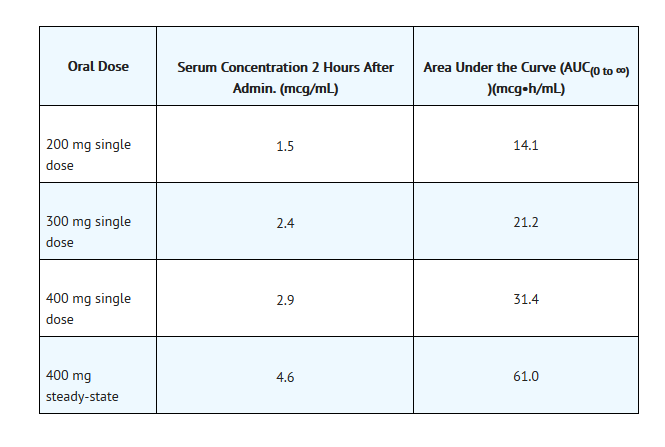
- Steady-state concentrations were attained after four oral doses, and the area under the curve (AUC) was approximately 40% higher than the AUC after single doses. Therefore, after multiple-dose administration of 200 mg and 300 mg doses, peak serum levels of 2.2 mcg/mL and 3.6 mcg/mL, respectively, are predicted at steady-state.
- In vitro, approximately 32% of the drug in plasma is protein bound.
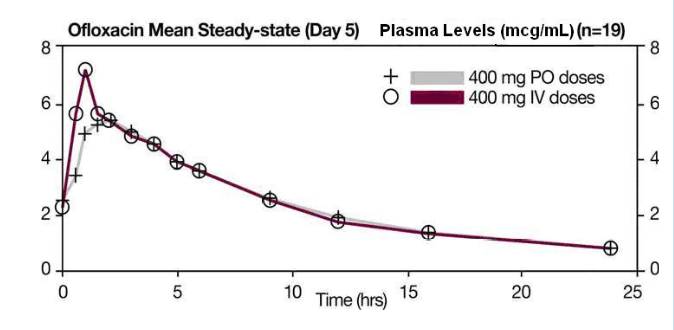
- The single dose and steady-state plasma profiles of ofloxacin injection were comparable in extent of exposure (AUC) to those of ofloxacin tablets when the injectable and tablet formulations of ofloxacin were administered in equal doses (mg/mg) to the same group of subjects. The mean steady-state AUC(0 to 12) attained after the intravenous administration of 400 mg over 60 min was 43.5 mcg•h/mL; the mean steady-state AUC(0 to 12) attained after the oral administration of 400 mg was 41.2 mcg•h/mL (two one-sided t-test, 90% confidence interval was 103 to 109). (see following chart).
- Between 0 and 6 h following the administration of a single 200 mg oral dose of ofloxacin to 12 healthy volunteers, the average urine ofloxacin concentration was approximately 220 mcg/mL. Between 12 and 24 hours after administration, the average urine ofloxacin level was approximately 34 mcg/mL.
- Following oral administration of recommended therapeutic doses, ofloxacin has been detected in blister fluid, cervix, lung tissue, ovary, prostatic fluid, prostatic tissue, skin, and sputum. The mean concentration of ofloxacin in each of these various body fluids and tissues after one or more doses was 0.8 to 1.5 times the concurrent plasma level. Inadequate data are presently available on the distribution or levels of ofloxacin in the cerebrospinal fluid or brain tissue.
- Ofloxacin has a pyridobenzoxazine ring that appears to decrease the extent of parent compound metabolism. Between 65% and 80% of an administered oral dose of ofloxacin is excreted unchanged via the kidneys within 48 hours of dosing. Studies indicate that less than 5% of an administered dose is recovered in the urine as the desmethyl or N-oxide metabolites. Four to eight percent of an ofloxacin dose is excreted in the feces. This indicates a small degree of biliary excretion of ofloxacin.
- The administration of ofloxacin tablets with food does not affect the Cmax and AUC∞ of the drug, but Tmax is prolonged.
- Clearance of ofloxacin is reduced in patients with impaired renal function (creatinine clearance rate < 50 mL/min), and dosage adjustment is necessary. (see PRECAUTIONS, Generaland DOSAGE AND ADMINISTRATION).
- Following oral administration to healthy elderly subjects (65 to 81 years of age), maximum plasma concentrations are usually achieved one to two hours after single and multiple twice-daily doses, indicating that the rate of oral absorption is unaffected by age or gender. Mean peak plasma concentrations in elderly subjects were 9 to 21% higher than those observed in younger subjects. Gender differences in the pharmacokinetic properties of elderly subjects have been observed. Peak plasma concentrations were 114% and 54% higher in elderly females compared to elderly males following single and multiple twice-daily doses. [This interpretation was based on study results collected from two separate studies.] Plasma concentrations increase dose-dependently with the increase in doses after single oral dose and at steady state. No differences were observed in the volume of distribution values between elderly and younger subjects. As in younger subjects, elimination is mainly by renal excretion as unchanged drug in elderly subjects, although less drug is recovered from renal excretion in elderly subjects. Consistent with younger subjects, less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites in the elderly. A longer plasma half-life of approximately 6.4 to 7.4 hours was observed in elderly subjects, compared with 4 to 5 hours for young subjects. Slower elimination of ofloxacin is observed in elderly subjects as compared with younger subjects which may be attributable to the reduced renal function and renal clearance observed in the elderly subjects. Because ofloxacin is known to be substantially excreted by the kidney, and elderly patients are more likely to have decreased renal function, dosage adjustment is necessary for elderly patients with impaired renal function as recommended for all patients. (see PRECAUTIONS, Generaland DOSAGE AND ADMINISTRATION).
MICROBIOLOGY
- Ofloxacin is a quinolone antimicrobial agent. The mechanism of action ofofloxacin and other fluoroquinolone antimicrobials involves inhibition ofbacterial topoisomerase IV and DNA gyrase (both of which are type IItopoisomerases), enzymes required for DNA replication, transcription, repairand recombination.
- Ofloxacinhas in vitro activity against a wide range of gram-negative andgram-positive microorganisms. Ofloxacin is often bactericidal at concentrationsequal to or slightly greater than inhibitory concentrations.
- Fluoroquinolones,including ofloxacin, differ in chemical structure and mode of action fromaminoglycosides, macrolides and β-lactam antibiotics, including penicillins.Fluoroquinolones may, therefore, be active against bacteria resistant to theseantimicrobials.
- Resistanceto ofloxacin due to spontaneous mutation in vitro is a rare occurrence(range: 10-9to 10-11). Although cross-resistance has been observedbetween ofloxacin and some other fluoroquinolones, some microorganismsresistant to other fluoroquinolones may be susceptible to ofloxacin.
- Ofloxacinhas been shown to be active against most strains of the followingmicroorganisms both in vitro and in clinical infections as described inthe INDICATIONS AND USAGE section:
- AerobicGram-Positive Microorganisms
- Staphylococcusaureus(methicillin-susceptible strains)
- Streptococcuspneumoniae (penicillin-susceptible strains)
- Streptococcus pyogenes
- Aerobic Gram-Negative Microorganisms
- Citrobacter (diversus)koseri
- Enterobacter aerogenes
- Escherichia coli
- Haemophilus influenzae
- Klebsiellapneumoniae
- Neisseriagonorrhoeae
- Proteusmirabilis
- Pseudomonas aeruginosa
- As with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairlyrapidly during treatment with ofloxacin.
- Other Microorganisms
- Chlamydia trachomatis
- The following in vitro data areavailable, but their clinical significance is unknown.
- Ofloxacin exhibitsin vitrominimum inhibitory concentrations (MIC values) of 2 mcg/mL or less against most(≥ 90%) strains of the following microorganisms; however, the safety andeffectiveness of ofloxacin in treating clinical infections due to thesemicroorganisms have not been established in adequate and well-controlledtrials.
- Aerobic Gram-Positive Microorganisms
- Staphylococcus epidermidis(methicillin-susceptiblestrains)
- Staphylococcus saprophyticus
- Streptococcus pneumoniae (penicillin-resistantstrains)
- AerobicGram-Negative Microorganisms
- Acinetobactercalcoaceticus
- Bordetellapertussis
- Citrobacterfreundii
- Enterobactercloacae
- Haemophilusducreyi
- Klebsiellaoxytoca
- Moraxellacatarrhalis
- Morganellamorganii
- Proteusvulgaris
- Providencia rettgeri
- Providencia stuartii
- Serratia marcescens
- Anaerobic Microorganisms
- Clostridium perfringes
- Other Microorganisms
- Chlamydia pneumoniae
- Gardnerella vaginalis
- Legionella pneumophila
- Mycoplasma hominis
- Mycoplasma pneumoniae
- Ureaplasma urealyticum
- Ofloxacin is not active against Treponema pallidum (see WARNINGS).
Susceptibility Tests
Dilution Techniques
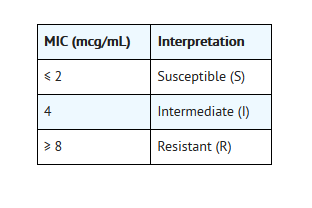
- Quantitative methods are used to determine antimicrobialminimum inhibitory concentrations (MIC values). These MIC values provideestimates of the susceptibility of bacteria to antimicrobial compounds. The MICvalues should be determined using a standardized procedure. Standardizedprocedures are based on a dilution method1,3(broth or agar) or equivalent with standardized inoculum concentrations andstandardized concentrations of ofloxacin powder. The MIC values should beinterpreted according to the following criteria:
- For testing Enterobacteriaceae,methicillin-susceptible Staphylococcus aureus, and Pseudomonas aeruginosa:
- For testing Haemophilusinfluenzae:a
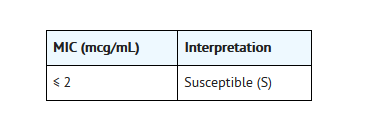
- aThisinterpretive standard is applicable only to broth microdilution susceptibilitytests with Haemophilus influenzae using HaemophilusTest Medium.1,3
- The current absence of data on resistant strains precludesdefining any results other than “Susceptible.” Strains yielding MIC resultssuggestive of a “nonsusceptible” category should be submitted to a referencelaboratory for further testing.
- For testing Neisseria gonorrhoeae:b
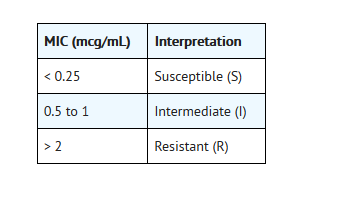
- bThese interpretive standards areapplicable only to agar dilution tests using GC agar base and 1% defined growthsupplement incubated in 5% CO2.
For testing Streptococcus pneumoniae and Streptococcus pyogenes:c
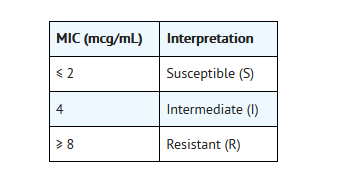
- c Theseinterpretive standards are applicable only to broth microdilutionsusceptibility tests using cation-adjusted Mueller-Hinton broth with 2 to 5%lysed horse blood.
- Areport of “Susceptible” indicates that the pathogen is likely to be inhibitedif the antimicrobial compound in the blood reaches the concentration usuallyachievable. A report of “Intermediate” indicates that the result should beconsidered equivocal, and, if the microorganism is not fully susceptible toalternative, clinically feasible drugs, the test should be repeated. Thiscategory implies possible clinical applicability in body sites where the drugis physiologically concentrated or in situations where a high dosage of drug canbe used. This category also provides a buffer zone which prevents smalluncontrolled technical factors from causing major discrepancies ininterpretation. A report of “Resistant” indicates that the pathogen is notlikely to be inhibited if the antimicrobial compound in the blood reaches theconcentration usually achievable; other therapy should be selected.
- Standardized susceptibility test procedures require the useof laboratory control microorganisms to control the technical aspects of thelaboratory procedures. Standard ofloxacin powder should provide the followingMIC values:
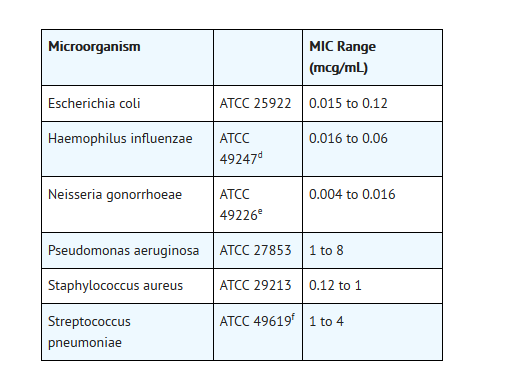
- dThisquality control range is applicable only to H. influenzae ATCC 49247 tested bya microdilution procedure using Haemophilus TestMedium (HTM).1,3
- e Thisquality control range is applicable only to N. gonorrhoeae ATCC 49226 tested byan agar dilution procedure using GC agar base with 1% defined growth supplementincubated in 5% CO2.
- f Thisquality control range is applicable only to S. pneumoniae ATCC 49619 tested bya microdilution procedure using cation-adjusted Mueller-Hinton broth with 2 to5% lysed horse blood.
DIffusion Techniques
- Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 5 mcg ofloxacin to test the susceptibility of microorganisms to ofloxacin.
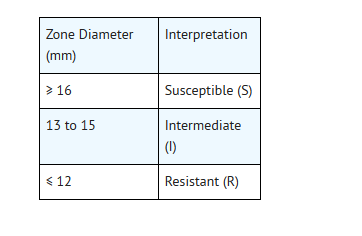
- Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5 mcg ofloxacin disk should be interpreted according to the following criteria:
- For testing Enterobacteriaceae, methicillin-susceptible Staphylococcus aureus, and Pseudomonas aeruginosa:
- For testing Haemophilus influenzae:g
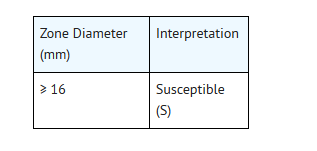
- g This zone diameter standard is applicable only to disk diffusiontests with Haemophilus influenzae using Haemophilus Test Medium (HTM)2incubatedin 5% CO2.
- The current absence of data on resistant strains precludesdefining any results other than “Susceptible.” Strains yielding zone diameterresults suggestive of a “nonsusceptible” category should be submitted to areference laboratory for further testing.
- For testing Neisseria gonorrhoeae: h
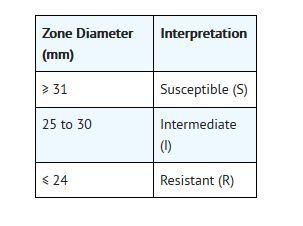
- h These zone diameter standards areapplicable only to disk diffusion tests using GC agar base and 1% definedgrowth supplement incubated in 5% CO2.
- For testing Streptococcus pneumoniaeandStreptococcus pyogenes: i
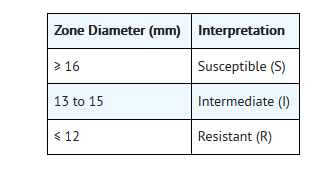
- iThese zone diameter standards areapplicable only to disk diffusion tests performed using Mueller-Hinton agarsupplemented with 5% defibrinated sheep blood and incubated in 5% CO2.
- Interpretation should be as stated above for results usingdilution techniques. Interpretation involves correlation of the diameterobtained in the disk test with the MIC for ofloxacin.
- As with standardized dilution techniques, diffusion methodsrequire the use of laboratory control microorganisms that are used to controlthe technical aspects of the laboratory procedures. For the diffusion technique,the 5 mcg ofloxacin disk should provide the following zone diameters in theselaboratory quality control strains:
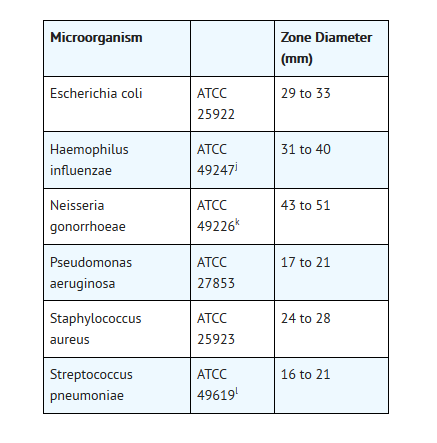
- jThisquality control range is applicable only to H. influenzaeATCC 49247 tested by a disk diffusion procedure using HaemophilusTest Medium (HTM)2 incubated in 5% CO2.
- k Thisquality control range is applicable only to N. gonorrhoeaeATCC 49226 tested by a disk diffusion procedure using GC agar base with 1%defined growth supplement incubated in 5% CO2.
- l Thisquality control range is applicable only to S. pneumoniaeATCC 49619 tested by a disk diffusion procedure using Mueller-Hinton agarsupplemented with 5% defibrinated sheep blood and incubated in 5% CO2.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies to determine the carcinogenic potential of ofloxacin have not been conducted.
- Ofloxacin was not mutagenic in the Ames bacterial test, in vitro and in vivo cytogenetic assay, sister chromatid exchange (Chinese Hamster and Human Cell Lines), unscheduled DNA Repair (UDS) using human fibroblasts, dominant lethal assays, or mouse micronucleus assay.
- Ofloxacin was positive in the UDS test using rat hepatocytes and Mouse Lymphoma Assay.
Animal Pharmacology
- Ofloxacin, as well as other drugs of the quinolone class, has been shown to cause arthropathies (arthrosis) in immature dogs and rats. In addition, these drugs are associated with an increased incidence of osteochondrosis in rats as compared to the incidence observed in vehicle-treated rats (see WARNINGS). There is no evidence of arthropathies in fully mature dogs at intravenous doses up to 3 times the recommended maximum human dose (on a mg/m2 basis or 5 times based on mg/kg basis), for a one-week exposure period.
- Long-term, high-dose systemic use of other quinolones in experimental animals has caused lenticular opacities; however, this finding was not observed in any animal studies with ofloxacin.
- Reduced serum globulin and protein levels were observed in animals treated with other quinolones. In one ofloxacin study, minor decreases in serum globulin and protein levels were noted in female cynomolgus monkeys dosed orally with 40 mg/kg ofloxacin daily for one year. These changes, however, were considered to be within normal limits for monkeys.
- Crystalluria and ocular toxicity were not observed in any animals treated with ofloxacin.
Clinical Studies
- There is limited information regarding Clinical Studies of Ofloxacin (oral) in the drug label.
How Supplied
- Ofloxacin tablets USP, 200 mg are available as light yellow to yellow, oval, biconvex film-coated tablets, debossed ‘CP110’ on one side and ‘200’ on other side. They are available in bottles of 50, 100 and 500 tablets.
- Bottles of 50 (NDC 65691-0104-1)
- Bottles of 100 (NDC 65691-0104-2)
- Bottles of 500 (NDC 65691-0104-3)
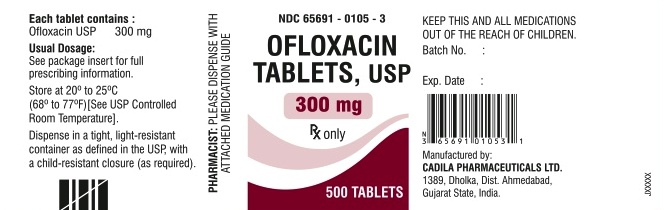
- Ofloxacin tablets USP, 300 mg are available as white to off-white, oval, biconvex film-coated tablets, debossed ‘CP109’ on one side and ‘300’ on other side. They are available in bottles of 50, 100 and 500 tablets.
- Bottles of 50 (NDC 65691-0105-1)
- Bottles of 100 (NDC 65691-0105-2)
- Bottles of 500 (NDC 65691-0105-3)
- Ofloxacin tablets USP, 400 mg are available as Yellow to dark yellow, oval, biconvex, film-coated tablets, debossed with ‘CP108’ on one side and ‘400’ on other side. They are available in bottles of 100 and 500 tablets.
- Bottles of 100 (NDC 65691-0106-1)
- Bottles of 500 (NDC 65691-0106-2)
Storage
- Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required)
- KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Images
Drug Images
{{#ask: Page Name::Ofloxacin (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Ofloxacin (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- Patients should be advised:
- to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue ofloxacin treatment. The risk of severe tendon disorders with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants;
- that fluoroquinolones like ofloxacin may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Patients should call their healthcare provider right away if you have any worsening muscle weakness or breathing problems;
- that antibacterial drugs including ofloxacin tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ofloxacin tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ofloxacin tablets or other antibacterial drugs in the future.
- that peripheral neuropathies have been associated with ofloxacin use, that symptoms may occur soon after initiation of therapy and may be irreversible.If symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness develop, they should discontinue ofloxacin and contact their physician;
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum- or magnesium-based antacids, sucralfate or didanosine chewable/buffered tablets or the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking ofloxacin (see Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e.g., dizziness, lightheadedness) and that patients should know how they react to ofloxacin before they operate an automobile or machinery or engage in activities requiring mental alertness and coordination (see WARNINGS and ADVERSE REACTIONS);
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (e.g., swelling of the lips, tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction (see WARNINGS and ADVERSE REACTIONS);
- that photosensitivity/phototoxicity has been reported in patients receiving quinolone antibiotics. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician (see PRECAUTIONS, GeneralandDrug Interactions);
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their physician before taking this drug if there is a history of this condition;
- that diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible;
- to inform their physician of any personal or family history of QTc prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any class IA (quinidine, procainamide), or class III (amiodarone, sotalol) antiarrhythmic agents.
- Patients should notify their physicians if they have any symptoms of prolongation of the QTc interval including prolonged heart palpitations or a loss of consciousness.

Precautions with Alcohol
- Alcohol-Ofloxacin (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Floxin, Ocuflox.
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ofloxacin (oral)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ofloxacin (oral) |Label Name=Ofloxacin (oral)11.png
}}
{{#subobject:
|Label Page=Ofloxacin (oral) |Label Name=Ofloxacin (oral)11.png
}}