Clevidipine dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Dosage and Administration
2.1 Monitoring
Monitor blood pressure and heart rate continually during infusion, and then until vital signs are stable. Patients who receive prolonged Cleviprex infusions and are not transitioned to other antihypertensive therapies should be monitored for the possibility of rebound hypertensionfor at least 8 hours after the infusion is stopped. These patients may need follow-up adjustments in blood pressure control.
2.2 Recommended Dosing
Cleviprex is intended for intravenous use. Titrate drug to achieve the desired blood pressure reduction. Individualize dosage depending on the blood pressure to be obtained and the response of the patient.
Initial dose: Initiate the intravenous infusion of Cleviprex at 1-2 mg/hour.
Dose titration: The dose may be doubled at short (90 second) intervals initially. As the blood pressure approaches goal, the increase in doses should be less than doubling and the time between dose adjustments should be lengthened to every 5-10 minutes. An approximately 1-2 mg/hour increase will generally produce an additional 2-4 mmHg decrease in systolic pressure.
Maintenance dose: The desired therapeutic response for most patients occurs at doses of 4-6 mg/hour. Patients with severe hypertensionmay require doses up to 32 mg/hour, but there is limited experience at this dose rate.
Maximum dose: Most patients were treated with maximum doses of 16 mg/hour or less. There is limited short-term experience with doses up to 32 mg/hour. Because of lipid load restrictions, no more than 1000 mL or an average of 21 mg/hour of Cleviprex infusion is recommended per 24 hour period. In clinical trials, 55 hypertensive patients were treated with > 500mL of Cleviprex infusion per 24 hour period. There is little experience with infusion durations beyond 72 hours at any dose.
Transition to an oral antihypertensive agent: Discontinue Cleviprex or titrate downward while appropriate oral therapy is established. When an oral antihypertensive agent is being instituted, consider the lag time of onset of the oral agent’s effect. Continue blood pressure monitoring until desired effect is achieved.
Special populations: Special populations were not specifically studied. In clinical trials, 78 patients with abnormal hepatic function (one or more of the following: elevated serum bilirubin, AST/SGOT, ALT/SGPT) and 121 patients with moderate to severe renal impairment were treated with Cleviprex. An initial Cleviprex infusion rate of 1-2 mg/hour is appropriate in these patients.
Table 1 is a guideline for dosing conversion from mg/hour to mL/hour.
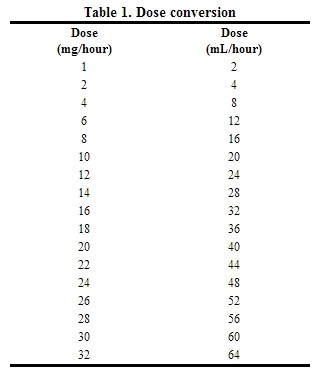 |
2.3 Instructions for Administration
Maintain aseptic technique while handling Cleviprex. Cleviprex is a single-use parenteral product. Do not use if contamination is suspected. Once the stopper is punctured, use within 12 hours and discard any unused portion.
Cleviprex is supplied in sterile, pre-mixed, ready-to-use 50 mL or 100 mL vials. Invert vial gently several times before use to ensure uniformity of the emulsion prior to administration. Inspect parenteral drug products for particulate matter and discoloration prior to administration whenever solution and container permit. Administer Cleviprex using an infusion device allowing calibrated infusion rates. Commercially available standard plastic cannulae may be used to administer the infusion.Administer Cleviprex by a central line or a peripheral line.
Cleviprex should not be administered in the same line as other medications.
Cleviprex should not be diluted, but it can be administered with the following:
Water for Injection, USP Sodium Chloride (0.9%) Injection, USP Dextrose (5%) Injection, USP Dextrose (5%) in Sodium Chloride (0.9%) Injection, USP Dextrose (5%) in Ringers Lactate Injection, USP Lactated Ringers Injection, USP 10% amino acid
References
- ↑ "CLEVIPREX (CLEVIDIPINE) EMULSION [THE MEDICINES COMPANY]". Retrieved 27 February 2014.