Diphyllobothrium
| style="background:#Template:Taxobox colour;"|Diphyllobothrium | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Proglottids of D. latum
| ||||||||||||||
| style="background:#Template:Taxobox colour;" | Scientific classification | ||||||||||||||
| ||||||||||||||
| Species | ||||||||||||||
|
D. latum |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
For details about infections caused by Diphyllobothrium, click here.
|
WikiDoc Resources for Diphyllobothrium |
|
Articles |
|---|
|
Most recent articles on Diphyllobothrium Most cited articles on Diphyllobothrium |
|
Media |
|
Powerpoint slides on Diphyllobothrium |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Diphyllobothrium at Clinical Trials.gov Trial results on Diphyllobothrium Clinical Trials on Diphyllobothrium at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Diphyllobothrium NICE Guidance on Diphyllobothrium
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Diphyllobothrium Discussion groups on Diphyllobothrium Patient Handouts on Diphyllobothrium Directions to Hospitals Treating Diphyllobothrium Risk calculators and risk factors for Diphyllobothrium
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Diphyllobothrium |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Diphyllobothrium is a genus of tapeworm which can cause Diphyllobothriasis in humans through consumption of raw or undercooked fish. The principal species causing diphyllobothriosis is Diphyllobothrium latum, known as the broad or fish tapeworm, or broad fish tapeworm. D. latum is a pseudophyllid cestode that infects fish and mammals. D. latum is native to Scandinavia, western Russia, and the Baltics, though it is now also present in North America, especially the Pacific Northwest. Other members of the genus Diphyllobothrium include Diphyllobothrium dendriticum (the salmon tapeworm), which has a much larger range (the whole northern hemisphere), D. pacificum, D. cordatum, D. ursi, D. lanceolatum, D. dalliae, and D. yonagoensis, all of which infect humans only infrequently. In Japan, the most common species in human infection is D. nihonkaiense, which was only identified as a separate species from D. latum in 1989.[1]
History
The fish tapeworm has a long documented history of infecting people who regularly consume fish and especially those whose customs include the consumption of raw or undercooked fish. In the 1970’s, most of the known cases of diphyllobothriasis came from Europe (5 million cases), and Asia (4 million cases) with fewer cases coming from North America and South America, and no reliable data on cases from Africa or Australia [2]. Interestingly, despite the relatively small number of cases seen today in South America, some of the earliest archeological evidence of diphyllobothriasis comes from sites in South America. Evidence of Diphyllobothrium spp. has been found in 4,000-10,000 year old human remains on the western coast of South America [3]. There is no clear point in time when Diphyllobothrium latum and related species were “discovered” in humans, but it is clear that diphyllobothriasis has been endemic in human populations for a very long time. Due to the changing dietary habits in many parts of the world, autochthonous, or locally-acquired, cases of diphyllobothriasis have recently been documented in previously non-endemic areas, such as Brazil [4]. In this way, diphyllobothriasis represents an emerging infectious disease in certain parts of the world where cultural practices involving eating raw or undercooked fish are being introduced
Morphology
The adult worm is comprised of three fairly distinct morphological segments; the scolex, the neck and the lower body. The scolex is the head portion of the worm, and is equipped with a slit-like groove (the bothrium) for attachment to the intestine. The scolex attaches to the neck, or proliferative region. From the neck, grows many proglottid segments which contain the reproductive organs of the worm. D. latum is the longest tapeworm in humans, averaging ten meters long. Adults can shed up to a million eggs a day. In adults, proglottids are wider than they are long (hence the name broad tapeworm). As in all pseudophyllid cestodes, the genital pores open midventrally.
Life cycle
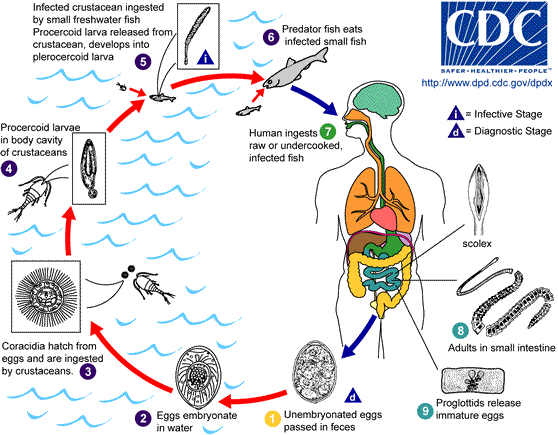
Adult tapeworms may infect humans, canids, felines, bears, pinnipeds, and mustelids, though the accuracy of the records for some of the nonhuman species is disputed. Immature eggs are passed in feces of the mammal host (the definitive host, where the worms reproduce). After ingestion by a suitable freshwater crustacean such as a copepod (the first intermediate host) the coracidia develop into procercoid larvae. Following ingestion of the copepod by a suitable second intermediate host, typically a minnow or other small freshwater fish, the procercoid larvae are released from the crustacean and migrate into the fish's flesh where they develop into a plerocercoid larvae (sparganum). The plerocercoid larvae are the infective stage for the definitive host (including humans).
Because humans do not generally eat undercooked minnows and similar small freshwater fish, these do not represent an important source of infection. Nevertheless, these small second intermediate hosts can be eaten by larger predator species, for example, trout, perch, and walleyed pike. In this case, the sparganum can migrate to the musculature of the larger predator fish and mammals can acquire the disease by eating these later intermediate infected host fish raw or undercooked. After ingestion of the infected fish, the plerocercoids develop into immature adults and then into mature adult tapeworms which will reside in the small intestine. The adults attach to the intestinal mucosa by means of the two bilateral grooves (bothria) of their scolex. The adults can reach more than 10 m (up to 30 ft) in length in some species such as D. latum, with more than 3,000 proglottids. One or several of the tape-like proglottid segments (hence the name tape-worm) regularly detach from the main body of the worm and release immature eggs in fresh water to start the cycle over again. Immature eggs are discharged from the proglottids (up to 1,000,000 eggs per day per worm) and are passed in the feces. The incubation period in humans, after which eggs begin to appear in the feces is typically 4-6 weeks, but can vary from as short as 2 weeks to as long as 2 years[5]. The tapeworm can live up to 20 years.
Clinical Symptoms
Symptoms of diphyllobothriasis are generally mild, and can include diarrhea, abdominal pain, vomiting, weight loss, fatigue, constipation and discomfort[6]. Approximately four out of five cases are asymptomatic and may go many years without being detected[7]. In a small number of cases, this leads to severe vitamin B12 deficiency due to the parasite absorbing 80% or more of the host’s B12 intake, and a megaloblastic anemia indistinguishable from pernicious anemia[8]. The anemica can also lead to subtle demyelinative neurological symptoms (subacute combined degeneration of spinal cord). Infection for many years is ordinarily required to deplete the human body of vitamin B-12 to the point that neurological symptoms appear.
Diagnosis
Diagnosis is usually made by identifying proglottid segments, or characteristic eggs in the feces[9]. These simple diagnostic techniques are able to identify the nature of the infection to the genus level, which is usually sufficient in a clinical setting [10]. However, when the species needs to be determined (in epidemiological studies, for example), restriction fragment length polymorphisms can be effectively used. PCR can be performed on samples of purified eggs, or native fecal samples following sonication of the eggs to release their contents [11].
Treatment
Upon diagnosis, treatment is quite simple and effective. The standard treatment for diphyllobothriasis, as well as many other tapeworm infections is a single dose of Praziquantel, 5-10 mg/kg PO once for both adults and children. An alternative treatment is Niclosamide, 2 g PO once for adults or 50 mg/kg PO once [12]. Another interesting potential diagnostic tool and treatment is the contrast medium, Gastrografin, introduced into the duodenum, which allows both visualization of the parasite, and has also been shown to cause detachment and passing of the whole worm [13].
Side Effects of Treatment
Praziquantel has few side effects, many of which are similar to the symptoms of diphyllobothriasis. They include malaise, headache, dizziness, abdominal discomfort, nausea, rise in temperature and occasionally allergic skin reactions [14]. The side effects of Niclosamide are very rare, due to the fact that it is not absorbed in the gastrointestinal tract [15].
Epidemiology
People at high risk for infection have traditionally been those who regularly consume raw fish, including fishermen who eat the raw liver or roe of their catches and women preparing and tasting foods that contain raw fish [16]. Many regional cuisines include raw or undercooked food, including sushi and sashimi in Japanese cuisine, carpaccio di persico in Italian, tartare maison in French-speaking populations, gefilte fish in Jewish populations, ceviche in Latin American cuisine. With emigration and globalization, the practice of eating raw fish in these and other dishes has brought diphyllobothriasis to new parts of the world and created new endemic foci of disease[17].
Public Health Strategies
The most viable interventions include: prevention of water contamination both by raising public awareness of the dangers of defecating in recreational bodies of water and by implementation of basic sanitation measures; screening and successful treatment of people infected with the parasite; and prevention of infection of humans via consumption of raw, infected fish [18]. The last of these can most easily be changed via education about proper preparation of fish. Fish that is thoroughly cooked, brined, or frozen at -10˚C for 24-48 hours can be consumed without risk of D. latum infection.
See also
http://www.stanford.edu/class/humbio103/parasites.htm
References
- "DPDx - Diphyllobothriasis". CDC Division of Parasitic Diseases.
- "UDiphyllobothrium spp". S FDA/CFSAN - Bad Bug Book.
- Janovy, John; Roberts, Larry S. (2005). Foundations of Parasitology (7th ed.). McGraw-Hill Education (ISE Editions). ISBN 0-07-111271-5.
- ↑ Lou YS, Koga M, Higo H; et al. (1989). "A human infection of the cestode, Diphyllobothrium nihonkaiense". Fukuoka Igaku Zasshi. 80: 446–50. PMID 2807129.
- ↑ Scholz, T; et al. (2009). "Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance". Clinical Microbiology Reviews. 22: 146–160. PMID 19136438.
- ↑ Reinhard, KJ (1992). "Parasitology as an interpretive tool in archaeology". American Antiquity. 57: 231–245.
- ↑ Llaguno, Mauricio M., et al. “Diphyllobothrium latum infection in a non-endemic country: case report.” (2008) Revista da Sociedade Brasileira de Medicina Tropical, 41 (3), 301-303
- ↑ http://web.gideononline.com/web/epidemiology/
- ↑ http://www.dpd.cdc.gov/dpdx/HTML/diphyllobothriasis.htm
- ↑ Sholz, et al. (2009)
- ↑ John, David T. and Petri, William A. (2006)
- ↑ http://web.gideononline.com/web/epidemiology/
- ↑ Sholz, et al. (2009)
- ↑ Sholz, et al. (2009)
- ↑ http://www.dpd.cdc.gov/dpdx/HTML/PDF_Files/MedLetter/TapewormInfection.pdf
- ↑ Ko, S.B. “Observation of deworming process in intestinal Diphyllobothrium latum parasitism by Gastrografin injection into jejunum through double-balloon enteroscope.” (2008) from Letter to the Editor; American Journal of Gastroenterology, 103; 2149-2150.
- ↑ Sholz, et al. (2009)
- ↑ Sholz, et al. (2009)
- ↑ Sholz, et al. (2009)
- ↑ Sholz, et al. (2009)
- ↑ Sholz, et al. (2009)
de:Fischbandwurm id:Cacing pita ikan la:Diphyllobothrium latum nl:Vislintworm fi:Lapamato sv:Bred binnikemask