Hepatitis B Vaccine, adjuvanted (Heplisav-B)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Hepatitis B Vaccine, adjuvanted (Heplisav-B) is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to HEPLISAV-B during pregnancy. Women who receive HEPLISAV-B during pregnancy are encouraged to contact 1-844-443-7734.
Risk Summary
- All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In clinically recognized pregnancies in the US general population, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20%.
- There are no clinical studies of HEPLISAV-B in pregnant women. Available human data on HEPLISAVB administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
- In a developmental toxicity study, 0.3 mL of a vaccine formulation containing 2.5 mcg HBsAg and 3000 mcg cytosine phosphoguanine (CpG) 1018 adjuvant was administered to female rats prior to mating and during gestation. These animal studies revealed no evidence of harm to the fetus due to this vaccine formulation.
Data (Animal)
- Developmental toxicity studies were conducted in female rats. Animals were administered 0.3 mL of a vaccine formulation containing 2.5 mcg HBsAg and 3000 mcg CpG 1018 adjuvant twice prior to mating, and on gestation days 6 and 18 (a single human dose of HEPLISAV-B contains 20 mcg HBsAg and 3000 mcg CpG 1018 adjuvant). No adverse effects on pre-natal and post-natal development up to the time of weaning were observed. There were no vaccine-related fetal malformations or variations observed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hepatitis B Vaccine, adjuvanted (Heplisav-B) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Hepatitis B Vaccine, adjuvanted (Heplisav-B) during labor and delivery.
Nursing Mothers
Risk Summary
- It is not known whether HEPLISAV-B is excreted in human milk. Data are not available to assess the effects of HEPLISAV-B on the breastfed infant or on milk production/excretion.
- The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for HEPLISAV-B and any potential adverse effects on the breastfed child from HEPLISAVB or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
- HEPLISAV-B is a clear to slightly opalescent, colorless to slightly yellow solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
- Administer HEPLISAV-B by intramuscular injection in the deltoid region using a sterile needle and syringe.
Monitoring
There is limited information regarding Hepatitis B Vaccine, adjuvanted (Heplisav-B) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Hepatitis B Vaccine, adjuvanted (Heplisav-B) and IV administrations.
Overdosage
There is limited information regarding Hepatitis B Vaccine, adjuvanted (Heplisav-B) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Hepatitis B Vaccine, adjuvanted (Heplisav-B)
| |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | J07 |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Infection with hepatitis B virus can have serious consequences including acute massive hepatic necrosis and chronic active hepatitis. Chronically infected persons are at increased risk for cirrhosis and hepatocellular carcinoma. Antibody concentrations ≥10 mIU/mL against HBsAg are recognized as conferring protection against hepatitis B virus infection.
Structure
There is limited information regarding Hepatitis B Vaccine, adjuvanted (Heplisav-B) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Hepatitis B Vaccine, adjuvanted (Heplisav-B) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Hepatitis B Vaccine, adjuvanted (Heplisav-B) Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- HEPLISAV-B has not been evaluated for carcinogenicity, mutagenic potential or male infertility in animals. Vaccination of female rats with a vaccine formulation containing 2.5 mcg HBsAg and 3000 mcg CpG 1018 adjuvant had no effect on fertility.
Clinical Studies
Evaluation of Seroprotection
- The immunogenicity of HEPLISAV-B was evaluated in comparison with a licensed hepatitis B vaccine (Engerix-B) in 3 randomized, active controlled, observer-blinded, multi-center Phase 3 clinical trials of adults. HEPLISAV-B was given as a 2-dose regimen at 0 and 1 months followed by saline placebo at 6 months. Engerix-B was given at 0, 1, and 6 months.
- The trials compared the seroprotection rates (% with antibody concentration ≥ 10 mIU/mL) induced by HEPLISAV-B and Engerix-B. Noninferiority was met if the lower bound of the 95% confidence interval of the difference in seroprotection rates (HEPLISAV-B minus Engerix-B) was greater than -10%.
Study 1: Seroprotection in Adults 18 through 55 Years of Age
- In Study 1, the immunogenicity population comprised 1511 participants who received HEPLISAV-B and 521 who received Engerix-B. The mean age was 40 years for both groups. The primary analysis compared the seroprotection rate at Week 12 for HEPLISAV-B with that at Week 28 for Engerix-B. Non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 3).
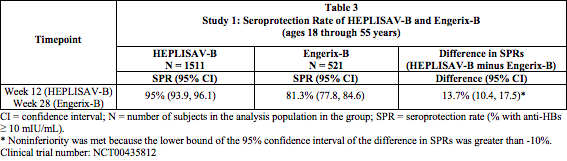
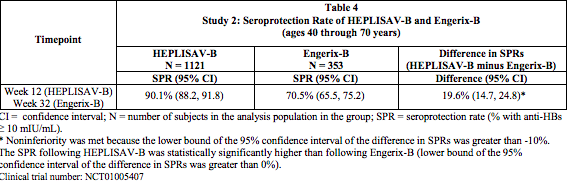
Study 2: Seroprotection in Adults 40 through 70 Years of Age
- In Study 2, the immunogenicity population comprised 1121 subjects who received HEPLISAV-B and 353 subjects who received Engerix-B. The mean age was 54 years for both groups. The primary analysis compared the seroprotection rate at Week 12 for HEPLISAV-B with that at Week 32 for Engerix-B. Noninferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 4).
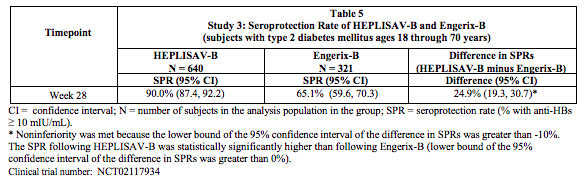
Study 3: Seroprotection in Adults 18 through 70 Years of Age Including those with Type 2 Diabetes Mellitus
- In Study 3, the immunogenicity population comprised 4537 subjects who received HEPLISAV-B and 2289 subjects who received Engerix-B. The mean age was 51 years and 14% of subjects had type 2 diabetes mellitus (defined as having a clinical diagnosis of type 2 diabetes and taking at least an oral or noninsulin injectable hypoglycemic agent and/or insulin).
- The primary analysis compared the seroprotection rate at Week 28 for HEPLISAV-B (n= 640) with that at Week 28 for Engerix-B (n= 321) in subjects with type 2 diabetes mellitus. Non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 5).
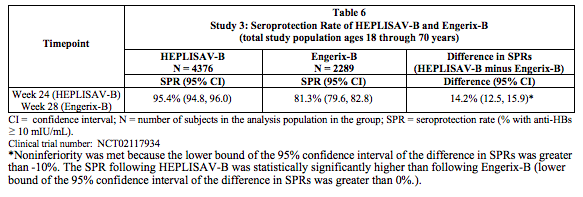
- A secondary analysis compared the seroprotection rate at Week 24 for HEPLISAV-B with that at Week 28 for Engerix-B in the total study population. Non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 6).
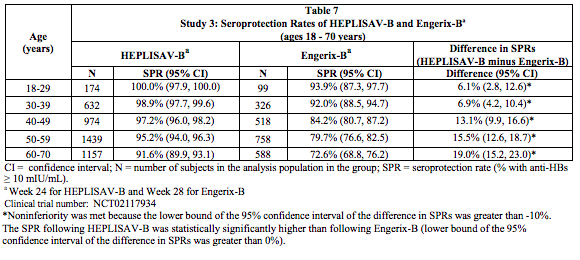
- Another secondary analysis compared the seroprotection rate at Week 24 for HEPLISAV-B with that at Week 28 for Engerix-B, by age group. For each age stratum non-inferiority of the seroprotection rate induced by HEPLISAV-B compared to Engerix-B was demonstrated (Table 7).
How Supplied
- Package of 5 single dose vials (NDC number: 43528-002-05)
- Package of 5 single dose prefilled syringes (NDC number: 43528-003-05) (packaged without needles)
- The tip caps and stoppers of the prefilled syringes and vial stoppers are not made with natural rubber latex.
Storage
- Store in a refrigerator at 2°C to 8°C (35°F to 46°F).
- Do not freeze; discard if the vaccine has been frozen.
- Do not use the vaccine after the expiration date shown on the vial or prefilled syringe label.
Images
Drug Images
{{#ask: Page Name::Hepatitis B Vaccine, adjuvanted (Heplisav-B) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
zzz {{#ask: Label Page::Hepatitis B Vaccine, adjuvanted (Heplisav-B) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform vaccine recipient of the potential benefits and risks associated with vaccination, as well as the importance of completing the immunization series.
- Emphasize that HEPLISAV-B contains non-infectious purified HBsAg and cannot cause hepatitis B infection.
- Advise vaccine recipient to report any adverse events to their healthcare provider or to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 and www.vaers.hhs.gov.
- Provide the Vaccine Information Statements, which are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Precautions with Alcohol
Alcohol-Hepatitis B Vaccine, adjuvanted (Heplisav-B) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Heplisav-B
Look-Alike Drug Names
There is limited information regarding Hepatitis B Vaccine, adjuvanted (Heplisav-B) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.