Aripiprazole (intramuscular): Difference between revisions
No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 182: | Line 182: | ||
|postmarketing=(Description) | |postmarketing=(Description) | ||
|drugInteractions=* Drug 1 | |drugInteractions=* Drug 1 | ||
*'''Ketoconazole and Other CYP3A4 Inhibitors''' | |||
* Drug 2 | * Drug 2 | ||
* Drug 3 | * Drug 3 | ||
Revision as of 13:17, 23 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDALITY AND ANTIDEPRESSANT DRUGS
See full prescribing information for complete Boxed Warning.
* Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis.
|
Overview
Aripiprazole (intramuscular) is an atypical antipsychotic that is FDA approved for the treatment of schizophrenia, bipolar I disorder, major depressive disorder, and irritability associated with autistic disorder. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, vomiting, constipation, headache, dizziness, akathisia, anxiety, insomnia, and restlessness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Schizophrenia
- Dosing Information
- The recommended starting and target dose for ABILIFY is 10 mg/day or 15 mg/day administered on a once-a-day schedule without regard to meals.
- Maintenance Treatment: Maintenance of efficacy in schizophrenia was demonstrated in a trial involving patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from those medications and randomized to either ABILIFY 15 mg/day or placebo, and observed for relapse. Patients should be periodically reassessed to determine the continued need for maintenance treatment.
Bipolar I Disorder
- Dosing Information
- (Dosage)
Adjunctive Treatment of Major Depressive Disorder
- Dosing Information
- (Dosage)
Irritability Associated with Autistic Disorder
- Dosing Information
- (Dosage)
Agitation Associated with Schizophrenia or Bipolar Mania (Intramuscular Injection)
- Dosing Information
- (Dosage)
Dosage Adjustment
Dosage adjustments in adults are not routinely indicated on the basis of age, gender, race, or renal or hepatic impairment status.
- Dosage adjustment for patients taking aripiprazole concomitantly with strong CYP3A4 inhibitors: When concomitant administration of aripiprazole with strong CYP3A4 inhibitors such as ketoconazole or clarithromycin is indicated, the aripiprazole dose should be reduced to one-half of the usual dose. When the CYP3A4 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should then be increased.
- Dosage adjustment for patients taking aripiprazole concomitantly with potential CYP2D6 inhibitors: When concomitant administration of potential CYP2D6 inhibitors such as quinidine, fluoxetine, or paroxetine with aripiprazole occurs, aripiprazole dose should be reduced at least to one-half of its normal dose. When the CYP2D6 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should then be increased. When adjunctive ABILIFY is administered to patients with major depressive disorder, ABILIFY should be administered without dosage adjustment.
- Dosing recommendation in patients taking aripiprazole concomitantly with strong CYP3A4 and CYP2D6 inhibitors: When concomitant administration of aripiprazole with strong inhibitors of CYP3A4 (such as ketoconazole or clarithromycin) and CYP2D6 (such as quinidine, fluoxetine, or paroxetine) is indicated, the aripiprazole dose should be reduced to one-quarter (25%) of the usual dose. When the CYP3A4 and/or CYP2D6 inhibitor is withdrawn from the combination therapy, the aripiprazole dose should be increased.
- Dosing recommendation in patients taking aripiprazole concomitantly with strong, moderate, or weak inhibitors of CYP3A4 and CYP2D6: Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (eg, a potent CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favorable clinical response.
- Dosing recommendation in patients who are classified as CYP2D6 poor metabolizers (PM): The aripiprazole dose in PM patients should initially be reduced to one-half (50%) of the usual dose and then adjusted to achieve a favorable clinical response. The dose of aripiprazole for PM patients who are administered a strong CYP3A4 inhibitor should be reduced to one-quarter (25%) of the usual dose.
- Dosage adjustment for patients taking potential CYP3A4 inducers: When a potential CYP3A4 inducer such as carbamazepine is added to aripiprazole therapy, the aripiprazole dose should be doubled. Additional dose increases should be based on clinical evaluation. When the CYP3A4 inducer is withdrawn from the combination therapy, the aripiprazole dose should be reduced to 10 mg to 15 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aripiprazole in adult patients.
Non–Guideline-Supported Use
Borderline personality disorder
- Dosing Information
- Aripiprazole 10-15 mg/day added to sertraline 100-200 mg/day[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Known hypersensitivity reaction to ABILIFY. Reactions have ranged from pruritus/urticaria to anaphylaxis.
Warnings
|
WARNINGS: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDALITY AND ANTIDEPRESSANT DRUGS
See full prescribing information for complete Boxed Warning.
* Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis.
|
Use in Elderly Patients with Dementia-Related Psychosis
Increased Mortality
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis
Cerebrovascular Adverse Events, Including Stroke
In placebo-controlled clinical studies (two flexible dose and one fixed dose study) of dementia-related psychosis, there was an increased incidence of cerebrovascular adverse events (eg, stroke, transient ischemic attack), including fatalities, in aripiprazole-treated patients (mean age: 84 years; range: 78-88 years). In the fixed-dose study, there was a statistically significant dose response relationship for cerebrovascular adverse events in patients treated with aripiprazole. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis.
Safety Experience in Elderly Patients with Psychosis Associated with Alzheimer's Disease
- In three, 10-week, placebo-controlled studies of aripiprazole in elderly patients with psychosis associated with Alzheimer's disease (n=938; mean age: 82.4 years; range: 56-99 years), the treatment-emergent adverse events that were reported at an incidence of ≥3% and aripiprazole incidence at least twice that for placebo were lethargy [placebo 2%, aripiprazole 5%], somnolence (including sedation) [placebo 3%, aripiprazole 8%], and incontinence (primarily, urinary incontinence) [placebo 1%, aripiprazole 5%], excessive salivation [placebo 0%, aripiprazole 4%], and lightheadedness [placebo 1%, aripiprazole 4%].
- The safety and efficacy of ABILIFY in the treatment of patients with psychosis associated with dementia have not been established. If the prescriber elects to treat such patients with ABILIFY, vigilance should be exercised, particularly for the emergence of difficulty swallowing or excessive somnolence, which could predispose to accidental injury or aspiration.
Clinical Worsening of Depression and Suicide Risk
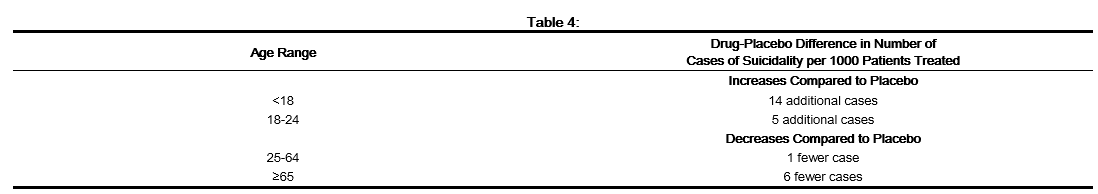
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term, placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18-24) with MDD and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
- The pooled analyses of placebo-controlled trials in children and adolescents with MDD, Obsessive Compulsive Disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 4.
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Ketoconazole and Other CYP3A4 Inhibitors
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Aripiprazole (intramuscular) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Aripiprazole (intramuscular) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Aripiprazole (intramuscular) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Aripiprazole interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Aripiprazole (intramuscular) Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Bellino S, Paradiso E, Bogetto F (2008). "Efficacy and tolerability of aripiprazole augmentation in sertraline-resistant patients with borderline personality disorder". Psychiatry Res. 161 (2): 206–12. doi:10.1016/j.psychres.2007.07.006. PMID 18848360.
- ↑ 2.0 2.1 2.2 2.3 2.4
- ↑ 3.0 3.1 3.2 3.3 3.4 "ABILIFY (aripiprazole) tablet ABILIFY (aripiprazole) solution ABILIFY DISCMELT (aripiprazole) tablet, orally disintegrating ABILIFY (aripiprazole) injection, solution [Otsuka America Pharmaceutical, Inc.]". DailyMed. Otsuka America Pharmaceutical, Inc. April 2013. Retrieved 22 October 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Abilify Tablets, Orodispersible Tablets, Oral Solution - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Otsuka Pharmaceuticals (UK) Ltd. 20 September 2013. Retrieved 22 October 2013.
- ↑ 5.0 5.1 5.2 5.3 5.4 "ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS" (PDF). European Medicines Agency. Otsuka Pharmaceutical Europe Ltd. Retrieved 22 October 2013.
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480008.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;008;10 |Pill Dosage=10 mg |Pill Color=Pink|+sep=; |Pill Shape= |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480008
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480006.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;006;2 |Pill Dosage=2 mg |Pill Color=Green|+sep=; |Pill Shape= |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480006
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480007.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;007;5 |Pill Dosage=5 mg |Pill Color=Blue|+sep=; |Pill Shape= |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480007
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480009.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;009;15 |Pill Dosage=15 mg |Pill Color=Yellow|+sep=; |Pill Shape=Round |Pill Size (mm)=6 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480009
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480010.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;010;20 |Pill Dosage=20 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=8 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480010
}}
{{#subobject:
|Page Name=Aripiprazole (intramuscular) |Pill Name=ABILIFY_NDC_591480011.jpg |Drug Name=ABILIFY |Pill Ingred=ARIPIPRAZOLE[ARIPIPRAZOLE]|+sep=; |Pill Imprint=A;011;30 |Pill Dosage=30 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Otsuka America Pharmaceutical, Inc. |NDC=591480011
}}