Digoxin immune fab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 329: | Line 329: | ||
* DigiFab is supplied as a sterile, purified, lyophilized preparation. Each vial contains 40 mg of digoxin immune Fab protein, contains no preservatives and is intended for one time use. | * DigiFab is supplied as a sterile, purified, lyophilized preparation. Each vial contains 40 mg of digoxin immune Fab protein, contains no preservatives and is intended for one time use. | ||
Each box contains 1 vial of DigiFab. | : Each box contains 1 vial of DigiFab. | ||
NDC 0281-0365-10 | : NDC 0281-0365-10 | ||
Storage Conditions: | |||
The product should be stored at 2° to 8°C (36° to 46°F). Do not freeze. The product must be used within 4 hours after reconstitution. | |||
* Storage Conditions | |||
: The product should be stored at 2° to 8°C (36° to 46°F). Do not freeze. The product must be used within 4 hours after reconstitution. | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
Revision as of 00:44, 5 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Digoxin immune fab is a Immunoglobulin G antidote that is FDA approved for the {{{indicationType}}} of potentially life-threatening digoxin toxicity or overdose. Common adverse reactions include hypokalemia, exacerbation of low cardiac output, and rapid ventricular response in patients with atrial fibrillation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Digitalis Toxicity
Dosage for Acute Ingestion of Unknown Amounts of Digoxin or Digitoxin
- If a patient presents with life-threatening digitalis toxicity caused by an acute ingestion and neither a serum digitalis concentration nor an estimated ingestion amount is available, 20 vials of DigiFab may be administered. This amount should be adequate to treat most life-threatening overdoses in adults and children. However, in small children it is important to monitor for volume overload. In general, a larger dose of DigiFab has a faster onset of effect but may enhance the possibility of a febrile reaction. In such cases, 10 vials may be administered first with careful monitoring of the patient's response followed at the physician's discretion by 10 additional vials and continued monitoring. Failure of the patient to respond to DigiFab should alert the physician to the possibility that the clinical problem may not be caused by digitalis toxicity.
Dosage for Toxicity During Chronic Therapy
- For adult patients who are in acute distress or for whom a serum digoxin concentration is not available, 6 vials (240 mg) should be adequate to reverse most cases of toxicity. For infants and small children (≤ 20 kg) on chronic therapy with digoxin and showing signs of toxicity, a single vial should be sufficient.
Dosage Calculation
- Methods for calculating a neutralizing dose of DigiFab, based on a known or estimated amount of digoxin or digitoxin in the body, are provided below. When using the dose calculation methods provided, the following guidelines should be considered:
- Inaccurate estimates of the amount of digitalis ingested or absorbed may occur due to non-steady state serum concentrations or due to digitalis assay limitations. Most serum digoxin assay kits are designed to measure concentrations less than 5 ng/mL, therefore sample dilution is required to accurately measure serum concentrations > 5 ng/mL.
- Dosage calculations are based on a steady state volume of distribution of approximately 5 L/kg for digoxin, which is used to convert serum digoxin concentrations to total body burden of digoxin in milligrams. The volume of distribution is a population average and may vary among individuals. Many patients may require higher doses for complete neutralization and doses should usually be rounded up to the nearest whole vial.
- If toxicity has not adequately reversed after several hours, or appears to recur, re-administration of DigiFab, at a dose guided by clinical judgment, may be necessary. If a patient is in need of re-administration of DigiFab due to recurrent toxicity, or to a new toxic episode that occurs soon after the first episode, measurement of free (unbound) serum digitalis concentrations should be considered since Fab may still be present in the body.
- Failure of a patient to respond to DigiFab treatment may indicate that the clinical problem is not caused by digitalis intoxication. If there is no response to an adequate dose of DigiFab, the diagnosis of digitalis toxicity should be questioned.
For Ingestion of Known Amount
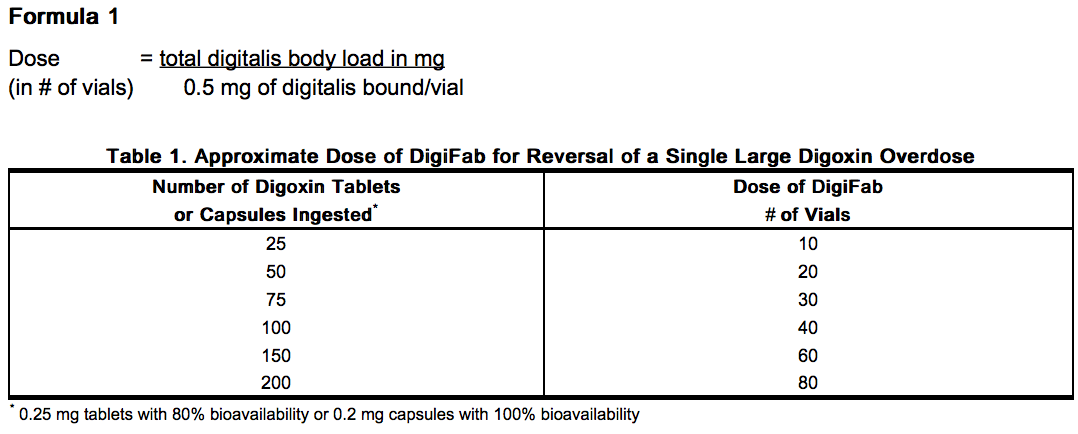
- Each vial of DigiFab contains 40 mg of purified digoxin-specific Fab, which will bind approximately 0.5 mg of digoxin. The total number of vials required can be calculated by dividing the total body load of digoxin in milligrams (mg) by 0.5 mg per vial (see Formula 1). Following an acute ingestion, total body load will be approximately equal to the amount ingested in milligrams for either digoxin capsules or digitoxin. If digoxin tablets were ingested, the total body load will be approximately equal to the amount ingested (in mg) multiplied by the bioavailability of the tablet preparation, which is 0.8.
- Table 1 gives dosage estimates in number of vials for adults and children who have ingested a single large dose of digoxin and for whom the approximate number of tablets or capsules is known. The dose of DigiFab (in number of vials) represented in Table 1 can be approximated using the following formula:
- If, after several hours, toxicity is not adequately reversed, or appears to recur, additional administration of DigiFab at a dose guided by clinical judgment may be required.
Calculations Based on Steady-State Serum Digoxin Concentrations
- Table 2 gives dosage estimates in number of vials for adult patients for whom a steady-state serum digoxin concentration is known. The dose of DigiFab (in number of vials) represented in Table 2 can be approximated using the following formula:
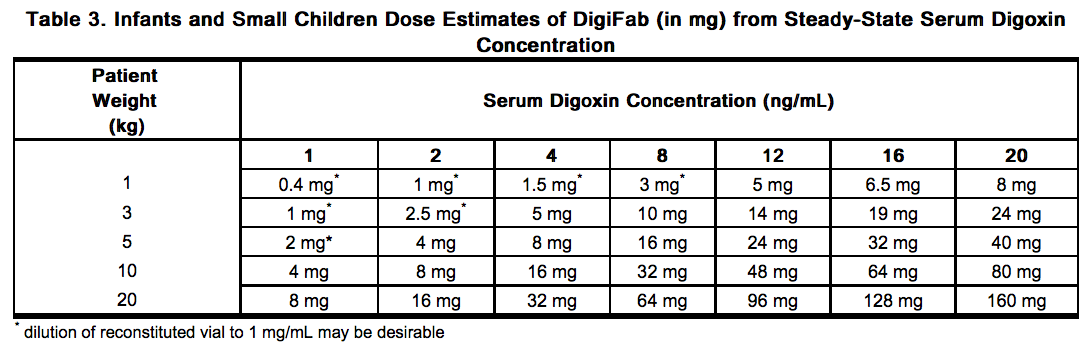
- Table 3 gives dosage estimates in milligrams for infants and small children based on the steady-state serum digoxin concentration. The dose of DigiFab represented in Table 3 can be estimated by multiplying the dose (in number of vials) calculated from Formula 2 by the amount of DigiFab contained in a vial (40 mg/vial) (see Formula 3). Since infants and small children can have much smaller dosage requirements, it is recommended that the 40 mg vial be reconstituted as directed and administered with a tuberculin syringe. For very small doses, a reconstituted vial can be diluted with 36 mL of sterile isotonic saline to achieve a concentration of 1 mg/mL.
Calculation Based on Steady-State Digitoxin Concentrations
- The dose of DigiFab for digitoxin toxicity can be approximated by using the following formula (which differs from Formula 2 in the denominator due to a 10-fold decrease in the volume of distribution of digitoxin as compared to digoxin).
- If in any case, the dose estimated based on ingested amount (Formula 1) differs substantially from that calculated based on the serum digoxin or digitoxin concentration (Formulas 2 and 4), it may be preferable to use the higher dose estimate.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Digoxin immune fab in adult patients.
Non–Guideline-Supported Use
Toxic Ingestions of Lanatoside C
- Dosing Information
- Fab fragments (500 mg) were added to 500 mL dextrose 5% in water and infused continuously at 32 drops per minute over a total period of 5.5 hours (total dose, 460 mg).[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Digoxin immune fab in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Digoxin immune fab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Digoxin immune fab in pediatric patients.
Contraindications
- There are no known contraindications to the use of DigiFab.
Warnings
- Suicidal ingestion may involve more than one drug. Toxic effects of other drugs or poisons should not be overlooked, especially in cases where signs and symptoms of digitalis toxicity are not relieved by administration of DigiFab.
- The possible risks and side-effects that attend the administration of heterologous animal proteins in humans include anaphylactic and anaphylactoid reactions, delayed allergic reactions and a possible febrile response to immune complexes formed by animal antibodies.8 Since the Fab fragment of the antibody lacks the antigenic determinants of the Fc fragment, it should pose a reduced immunogenic threat to patients compared with intact immunoglobulin molecules. Being monovalent, the product is also unlikely to form extended immune complexes with the antigen. Although no patient in the clinical studies of DigiFab has experienced a severe anaphylactic reaction, the possibility of an anaphylactic reaction should be considered. All patients should be informed of the possibility of an anaphylactic reaction and when receiving DigiFab should be carefully monitored for signs and symptoms of an acute allergic reaction (e.g., urticaria, pruritus, erythema, angioedema, bronchospasm with wheezing or cough, stridor, laryngeal edema, hypotension, tachycardia) and treated immediately with appropriate emergency medical care (e.g., oxygen, diphenhydramine, corticosteroids, volume expansion and airway management). If an anaphylactic reaction occurs during the infusion, DigiFab administration should be terminated at once and appropriate treatment administered. The need for epinephrine should be balanced against its potential risk in the setting of digitalis toxicity. Patients with known allergies to sheep protein would be particularly at risk for an anaphylactic reaction, as would individuals who have previously received intact ovine antibodies or ovine Fab.
- Papain is used to cleave the whole antibody into Fab and Fc fragments, and trace amounts of papain or inactivated papain residues may be present in DigiFab. Patients with allergies to papain, chymopapain, other papaya extracts, or the pineapple enzyme bromelain may also be at risk for an allergic reaction to DigiFab. In addition, it has been noted in the literature that some dust mite allergens and some latex allergens share antigenic structures with papain and patients with these allergies may be allergic to papain.9-10 DigiFab should not be administered to patients with a known history of hypersensitivity to papaya or papain unless the benefits outweigh the risks and appropriate management for anaphylactic reactions is readily available.
- Skin testing has not proved useful in predicting allergic response to Digibind.11 Because of this, and because it may delay urgently needed therapy, skin testing was not performed during the clinical studies of DigiFab and is not suggested prior to dosing with this product.
Precautions
- General
- Standard management of digitalis intoxication includes withdrawal of the intoxicating agent, correction of electrolyte disturbances (especially hyperkalemia), acid-base imbalances, hypoxia and treatment of cardiac arrhythmias.
- Massive digitalis intoxication can cause hyperkalemia; administration of potassium supplements in the setting of digitalis intoxication may be hazardous. After treatment with DigiFab, the serum potassium concentration may drop rapidly and must be monitored frequently, especially after the first several hours after DigiFab is given.
- Patients with poor cardiac function may deteriorate secondary to the withdrawal of the inotropic action of digoxin by DigiFab. If needed, additional support can be provided by using other intravenous inotropes such as dopamine, dobutamine or vasodilators. However, care must be taken not to aggravate the digitalis induced rhythm disturbances. Re-digitalization should be postponed, if possible, until the Fab fragments have been eliminated from the body, which may require several days, and patients with impaired renal function may require a week or longer.
- Use of DigiFab in Renal Failure
- The elimination half-life of DigiFab in renal failure has not been clearly defined, although patients with renal dysfunction have been successfully treated with Digibind.3,12 There is no evidence to suggest that the time-course of therapeutic effect is any different in these patients than in patients with normal renal function, but excretion of the Fab fragment-digoxin complex from the body is probably delayed. There is one case report of recurrence of atrioventricular block due to digoxin in a functionally anephric patient 10 days after its initial reversal by ovine Fab therapy.12 This clinical event persisted for more than a week. In patients that are functionally anephric, failure to clear the Fab-digoxin complex from the blood by glomerular filtration and renal excretion may be anticipated. It is uncertain whether the failure to eliminate the Fab-digoxin complex in severe renal impairment may lead to re-intoxication with digoxin following the release of previously bound digoxin into the blood. However, patients with severe renal failure who receive DigiFab for digitalis toxicity should be monitored for a prolonged period for possible recurrence of toxicity. Monitoring of free (unbound) digoxin concentrations after the administration may be appropriate in order to establish recrudescent toxicity in renal failure patients.
- Formation of Antibodies to DigiFab:
- Prior treatment with digoxin-specific ovine immune Fab carries a theoretical risk of sensitization to ovine serum protein and possible diminution of the efficacy of the drug due to the presence of human antibodies against ovine Fab. Human antibodies to ovine Fab have been reported in some patients receiving Digibind, however, to date, there have been no clinical reports of human anti-ovine immunoglobulin antibodies causing a reduction in binding of ovine digoxin immune Fab or neutralization response to ovine digoxin immune Fab.
- Laboratory Tests:
- DigiFab will interfere with digitalis immunoassay measurements in the same way that has been reported for Digibind.14,15 Thus, standard serum digoxin concentration measurements may be clinically misleading until the Fab fragments are eliminated from the body. This may take several days or a week or more in patients with markedly impaired renal function. Therefore, serum samples for digoxin concentration should be obtained before DigiFab administration, if at all possible. Such measurements would establish the level of serum digoxin at the time of diagnosis of digitalis intoxication. At least 6 to 8 hours are required for equilibration of digoxin between serum and tissue, so absorption of the last dose may continue from the intestine. Therefore, serum measurements may be difficult to interpret if samples are drawn soon after the last digitalis dose. Patients should be closely monitored, including temperature, blood pressure, electrocardiogram, and potassium concentration, during and after administration of DigiFab. The total serum digoxin concentration may rise precipitously following administration of DigiFab, but this will be almost entirely bound to the Fab fragment and therefore not able to react with receptors in the body.
- Digoxin causes a shift of potassium from inside to outside the cell, such that severe intoxication can cause a life-threatening elevation of serum potassium. This may lead to increased urinary excretion of potassium so that a patient may have hyperkalemia but a whole body deficit of potassium. When the toxic effects of digoxin are reversed by DigiFab, potassium shifts back into the cell with a resulting decline in serum potassium concentration. This hypokalemia may develop rapidly. For these reasons, serum potassium concentration should be followed closely, especially during the first several hours after DigiFab administration. Cautious potassium supplementation should then be given when necessary.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Digoxin immune fab in the drug label.
Postmarketing Experience
- Based on experience with Digibind, the following adverse reactions could occur with the use of DigiFab:
- Exacerbation of low cardiac output states and congestive heart failure due to the withdrawal of inotropic effect of digitalis.
- Hypokalemia due to reactivation of the sodium-potassium ATPase.
- Rapid ventricular response in patients with atrial fibrillation due to withdrawal of the effects of digitalis on the atrioventricular node.
- Rare allergic reactions. Patients with a history of allergy, especially to antibiotics, appear to be at particular risk.
- In the clinical trials of DigiFab, 6 of 15 patients in the digoxin overdose study had a total of 17 adverse experiences, most were mild to moderate in nature and all were deemed "remotely associated" with DigiFab. Three events were deemed "severe", all occurred in one patient and consisted of the following: pulmonary edema, bilateral pleural effusion and renal failure. After reviewing the case, it was determined that these events were likely due to the loss of digoxin inotropic support in combination with the patient's underlying medical condition. Of 8 healthy volunteers who received DigiFab, only 2 experienced an adverse reaction that was considered to be associated with DigiFab. The reactions were 1 episode of phlebitis of the infusion vein and 1 episode of moderate postural hypotension, which became mild prior to resolving.
Drug Interactions
- Studies of drug interactions have not been conducted with DigiFab.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Animal reproduction studies have not been conducted with DigiFab. It is also not known whether DigiFab can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. DigiFab should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Digoxin immune fab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Digoxin immune fab during labor and delivery.
Nursing Mothers
- It is not known whether DigiFab is excreted in human breast milk. Because many drugs are excreted in human milk, caution should be exercised when DigiFab is administered to a nursing woman.
Pediatric Use
- Specific studies in pediatric patients have not been conducted and no pediatric patients were enrolled in the clinical studies of DigiFab. A similar digoxin ovine Fab product, Digibind, has been used successfully to treat infants.2 As with all drugs, the use of DigiFab in infants and children should be based on careful consideration of the benefits compared with the potential risks.
Geriatic Use
- Specific studies in elderly patients have not been conducted. Of the 15 patients given DigiFab for digoxin toxicity in one clinical trial, the average age of all patients was 64 years and over half of the patients (8 of the 15) were 65 years of age or older. The oldest patient studied was 86 years old. There is no evidence that the efficacy of DigiFab would be altered due to advanced age alone, however elderly patients have a higher chance of having impaired renal function and therefore should be monitored more closely for recurrent toxicity.
Gender
There is no FDA guidance on the use of Digoxin immune fab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Digoxin immune fab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Digoxin immune fab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Digoxin immune fab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Digoxin immune fab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Digoxin immune fab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
- Each vial of DigiFab should be reconstituted with 4 mL of Sterile Water for Injection USP and gently mixed to provide a solution containing approximately 10 mg/mL of digoxin immune Fab protein. The reconsituted product should be used promptly. If not used immediately, it may be stored under refrigeration at 2° to 8°C (36° to 46°F} for up to 4 hours. The reconstituted product may be added to an appropriate volume of 0.9% sodium chloride for injection.
- DigiFab should be administered slowly as an intravenous infusion over at least 30 minutes. If infusion rate-related reactions occur, the infusion should be stopped and re-started at a slower rate. If cardiac arrest is imminent, DigiFab can be given by bolus injection. With bolus injection, an increased incidence of infusion-related reactions may be expected.
- For infants and small children who may require very small doses, it is recommended that the 40 mg vial be reconstituted as directed and administered undiluted using a tuberculin syringe. For very small doses, a reconstituted vial can be diluted with an additional 36 mL of isotonic saline to achieve a concentration of 1 mg/mL.
Monitoring
- All patients should be informed of the possibility of an anaphylactic reaction and when receiving DigiFab should be carefully monitored for signs and symptoms of an acute allergic reaction (e.g., urticaria, pruritus, erythema, angioedema, bronchospasm with wheezing or cough, stridor, laryngeal edema, hypotension, tachycardia) and treated immediately with appropriate emergency medical care (e.g., oxygen, diphenhydramine, corticosteroids, volume expansion and airway management).
- Patients should be closely monitored, including temperature, blood pressure, electrocardiogram, and potassium concentration, during and after administration of DigiFab.
- Patients with severe renal failure who receive DigiFab for digitalis toxicity should be monitored for a prolonged period for possible recurrence of toxicity. Monitoring of free (unbound) digoxin concentrations after the administration may be appropriate in order to establish recrudescent toxicity in renal failure patients.
IV Compatibility
There is limited information regarding IV Compatibility of Digoxin immune fab in the drug label.
Overdosage
Acute Overdose
- The maximum amount of DigiFab that can safely be administered in single or multiple doses has not been determined.
Chronic Overdose
There is limited information regarding Chronic Overdose of Digoxin immune fab in the drug label.
Pharmacology
Digoxin immune fab
| |
| Systematic (IUPAC) name | |
| Anti-digoxin antibody fragment | |
| Identifiers | |
| CAS number | ? |
| ATC code | V03 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 144602.257 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 15 hours for DigiFab, 23 hours for Digibind |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | IV infusion, injection |
Mechanism of Action
- DigiFab has an affinity for digoxin in the range of 109 to 1010 M-1, which is greater than the affinity of digoxin for its sodium pump receptor, the presumed receptor for its therapeutic and toxic effects. When administered to the intoxicated patient, DigiFab binds to molecules of digoxin reducing free digoxin levels, which results in a shift in the equilibrium away from binding to the receptors, thereby reducing cardio-toxic effects. Fab-digoxin complexes are then cleared by the kidney and reticuloendothelial system.
Structure
- DigiFab® [Digoxin Immune Fab (Ovine)] is a sterile, purified, lyophilized preparation of digoxin-immune ovine Fab (monovalent) immunoglobulin fragments. These fragments are obtained from the blood of healthy sheep immunized with a digoxin derivative, digoxin-dicarboxymethoxylamine (DDMA), a digoxin analogue which contains the functionally essential cyclopentaperhydrophenanthrene:lactone ring moiety coupled to keyhole limpet hemocyanin (KLH).
- The final product is prepared by isolating the immunoglobulin fraction of the ovine serum, digesting it with papain and isolating the digoxin-specific Fab fragments by affinity
chromatography. These antibody fragments have a molecular weight of approximately 46,000 Da.
- Each vial of DigiFab, which will bind approximately 0.5 mg digoxin, contains 40 mg of digoxin immune Fab, 75 mg (approx) of mannitol USP, and 2 mg (approx) sodium acetate USP as a buffering agent.
- The product contains no preservatives and is intended for intravenous administration after reconstitution with 4 mL of Sterile Water for Injection USP.
Pharmacodynamics
- No toxic effects were observed when DigiFab was administered to healthy male Sprague Dawley rats in equimolar doses sufficient to neutralize a 1 mg/kg dose of digoxin. In these studies, the physiologic changes produced by toxic serum concentrations of digoxin were ameliorated rapidly by the administration of DigiFab, or another ovine digoxin-specific immune Fab, Digibind® (manufactured by GlaxoSmithKline). Statistically equivalent responses were observed with both DigiFab and Digibind to the following variables: PTQ index, heart rate, mean arterial pressure, ventilation, arterial blood gases, and serum potassium concentrations.
Pharmacokinetics
- The pharmacokinetics of DigiFab were assessed in a randomized and controlled study of DigiFab and Digibind (comparator Fab product for treatment of digoxin toxicity). Sixteen healthy subjects were given 1 mg of intravenous digoxin followed by an approximately equimolar neutralizing dose of either DigiFab (n=8) or Digibind (n=8). The pharmacokinetic profiles of Fab were similar for both products.1 The similar volumes of distribution (0.3 L/kg and 0.4 L/kg for DigiFab and Digibind, respectively) indicate considerable penetration from the circulation into the extracellular space and are consistent with previous reports of ovine Fab distribution, as are the elimination half-life values (15 hours and 23 hours for DigiFab and Digibind, respectively).2-6 The elimination half-life of 15-20 hours in patients with normal renal function appears to be increased up to 10 fold in patients with renal impairment, although volume of distribution remains unaffected.6
Nonclinical Toxicology
- Animal carcinogenicity and reproduction studies have not been conducted with DigiFab.
Clinical Studies
- There have been two controlled clinical trials conducted with DigiFab: a pharmacokinetic and pharmacodynamic study of DigiFab as compared to Digibind in healthy volunteers, and a prospective multi-center study of the efficacy of DigiFab in patients presenting with life-threatening digoxin toxicity.
- The objective of the pharmacokinetic and pharmacodynamic study was to compare these parameters for DigiFab to those for Digibind.1 This trial was conducted in healthy volunteers who were administered a 1 mg intravenous dose of digoxin, followed 2 hours later by an equimolar neutralizing dose of either DigiFab or Digibind. The pharmacokinetics of both digoxin and Fab were determined. The primary outcome measure was the serum level of free (unbound) digoxin. The results demonstrated that both products reduced the level of free digoxin in the serum to below the limit of assay quantitation for several hours after Fab administration. Cumulative urinary excretion of digoxin was comparable for both products and exceeded 40% of the administered dose by 24 hours. These results demonstrate that DigiFab and Digibind have equivalent pharmacodynamic effects on the digoxin parameters that are relevant to the treatment of digoxin toxicity. In this study, no patients developed a measurable immune response (human anti-ovine antibodies) to DigiFab.
- The objective of the efficacy study was to demonstrate safety and also to determine the pharmacokinetics of, and clinical response to, DigiFab in patients. Results were compared to historical data on another U.S. marketed ovine digoxin immune Fab product, Digibind. Fifteen patients received doses of DigiFab based on its theoretical binding capacity for digoxin, and based on the known amount of digoxin ingested or on blood concentrations of digoxin at the time of admission. This study was conducted in both the U.S. and in Finland.
- The primary outcome of the study was met in that serum free digoxin concentrations in all patients fell to undetectable levels following DigiFab administration. This was an expected outcome that is consistent with data in the literature showing that free digoxin concentrations fall rapidly following administration of Digibind.2 In the DigiFab trial, an independent blinded review of each patient's ECG showed that 10 of the 15 patients studied had ECG abnormalities that improved within 4 hours after the DigiFab infusion. The remaining 5 patients had ECG abnormalities that were unchanged from baseline throughout the 24-hour assessment period, and in one case through the 30-day follow up period. Although the reason for the lack of ECG resolution could not be clearly determined in all cases, it is possible that the ECG abnormalities observed in these patients were not entirely due to digoxin toxicity, but rather to another underlying cardiac problem. Assessing all manifestations of toxicity, investigators classified 7 out of the 15 patients (47%) studied as having complete resolution of digoxin toxicity within 4 hours of DigiFab administration, and 14 patients (93%) were classified as having resolved their digoxin toxicity by 20 hours. The data for the proportion of patients who responded to treatment with DigiFab is similar to, and consistent with, historical data available for Digibind.2-3 In this study, where 2/15 patients had serum available for human anti-ovine antibody determination, there was no measurable immune response.
How Supplied
- DigiFab is supplied as a sterile, purified, lyophilized preparation. Each vial contains 40 mg of digoxin immune Fab protein, contains no preservatives and is intended for one time use.
- Each box contains 1 vial of DigiFab.
- NDC 0281-0365-10
- Storage Conditions
- The product should be stored at 2° to 8°C (36° to 46°F). Do not freeze. The product must be used within 4 hours after reconstitution.
Storage
There is limited information regarding Digoxin immune fab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Digoxin immune fab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Digoxin immune fab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be advised to contact their physician immediately if they experience any signs and symptoms of delayed allergic reactions or serum sickness (e.g., rash, pruritus, urticaria) after hospital discharge.
Precautions with Alcohol
- Alcohol-Digoxin immune fab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Digibind®[2]
- Digifab®[3]
Look-Alike Drug Names
- N/A[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Hess, T. (1979-12). "Treatment of a case of lanatoside C intoxication with digoxin-specific F(ab')2 antibody fragments". American Heart Journal. 98 (6): 767–771. ISSN 0002-8703. PMID 495429. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "DIGIFAB (ovine digoxin immune fab) injection, powder, lyophilized, for solution".
- ↑ "DIGIFAB (ovine digoxin immune fab) injection, powder, lyophilized, for solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Digoxin immune fab |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Digoxin immune fab |Label Name=Digoxin immune fab07.png
}}
{{#subobject:
|Label Page=Digoxin immune fab |Label Name=Digoxin immune fab08.png
}}