Bivalirudin clinical studies: Difference between revisions
(Created page with "__NOTOC__ {{Bivalirudin}} {{CMG}} {{AE}}{{JH}} ==Title== <ref>{{Cite web | last = | first = | title = ANGIOMAX (BIVALIRUDIN) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTIO...") |
No edit summary |
||
| Line 3: | Line 3: | ||
{{CMG}} {{AE}}{{JH}} | {{CMG}} {{AE}}{{JH}} | ||
== | ==Clinical Studies== | ||
====14.1 PCI/PTCA==== | |||
Angiomax has been evaluated in five randomized, controlled interventional cardiology trials reporting 11,422 patients. Stents were deployed in 6062 of the patients in these trials - mainly in trials performed since 1995. Percutaneous transluminal coronary angioplasty, atherectomy or other procedures were performed in the remaining patients. | |||
<ref>{{Cite web | last = | first = | title = ANGIOMAX (BIVALIRUDIN) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION [THE MEDICINES COMPANY] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=911cd48f-01ea-4dec-b30c-95e7e0ea9d2a | publisher = | date = | accessdate = 10 March 2014 }}</ref> | <u>REPLACE-2 Trial</u> | ||
This was a randomized, double-blind, multicenter study reporting 6002 (intent-to-treat) patients undergoing PCI. Patients were randomized to treatment with Angiomax with the "provisional" use of platelet glycoprotein IIb/IIIa inhibitor (GPI) or heparin plus planned use of GPI. GPIs were added on a "provisional" basis to patients who were randomized to Angiomax in the following circumstances: | |||
* decreased TIMI flow (0 to 2) or slow reflow; | |||
* dissection with decreased flow; | |||
* new or suspected thrombus; | |||
* persistent residual stenosis; | |||
* distal embolization; | |||
* unplanned stent; | |||
* suboptimal stenting; | |||
* side branch closure; | |||
* abrupt closure; clinical instability; and | |||
* prolonged ischemia. | |||
During the study, one or more of these circumstances occurred in 12.7% of patients in the Angiomax with provisional GPI arm. GPIs were administered to 7.2% of patients in the Angiomax with provisional GPI arm (62.2% of eligible patients). | |||
Patients ranged in age from 25-95 years (median, 63); weight ranged from 35-199 kg (median 85.5); 74.4% were male and 25.6% were female. Indications for PCI included unstable angina (35% of patients), myocardial infarction within 7 days prior to intervention (8% of patients), stable angina (25%) and positive ischemic stress test (24%). Stents were deployed in 85% of patients. Ninety-nine percent of patients received aspirin and 86% received thienopyridines prior to study treatment. | |||
Angiomax was administered as a 0.75 mg/kg bolus followed by a 1.75 mg/kg/h infusion for the duration of the procedure. The activated clotting time (ACT - measured by a Hemochron® device) was measured 5 min after the first bolus of study medication. If the ACT was <225 seconds, an additional bolus of 0.3 mg/kg was given. At investigator discretion, the infusion could be continued following the procedure for up to 4 hours. The median infusion duration was 44 min. Heparin was administered as a 65 U/kg bolus. The activated clotting time (ACT - measured by a Hemochron® device) was measured 5 min after the first bolus of study medication. If the ACT was <225 seconds, an additional bolus of 20 units/kg was given. GPIs (either abciximab or eptifibatide) were given according to manufacturers' instructions. Both randomized groups could be given "provisional" treatments during the PCI at investigator discretion, but under double-blind conditions. "Provisional" treatment with GPI was requested in 5.2% of patients randomized to heparin plus GPI (they were given placebo) and 7.2% patients randomized to Angiomax with provisional GPI (they were given abciximab or eptifibatide according to pre-randomization investigator choice and patient stratification). | |||
The percent of patients reaching protocol-specified levels of anticoagulation was greater in the Angiomax with provisional GPI group than in the heparin plus GPI group. For patients randomized to Angiomax with provisional GPI, the median 5 min ACT was 358 sec (interquartile range 320-400 sec) and the ACT was <225 sec in 3%. For patients randomized to heparin plus GPI, the median 5 min ACT was 317 sec (interquartile range 263-373 sec) and the ACT was <225 sec in 12%. At the end of the procedure, median ACT values were 334 sec (Angiomax group) and 276 sec (heparin plus GPI group). | |||
For the composite endpoint of death, MI, or urgent revascularization adjudicated under double-blind conditions, the frequency was higher (7.6%)(95% confidence interval 6.7%-8.6%) in the Angiomax with "provisional" GPI arm when compared to the heparin plus GPI arm (7.1%)(95% confidence interval 6.1%-8.0%). However, major hemorrhage was reported significantly less frequently in the Angiomax with provisional GPI arm (2.4%) compared to the heparin plus GPI arm (4.1%). Study outcomes are shown in Table 7. | |||
{| | |||
| [[File:Bivalirudin_08.png|thumb|600px]] | |||
|} | |||
At 12 months' follow-up, mortality was 1.9% among patients randomized to Angiomax with "provisional" GPIs and 2.5% among patients randomized to heparin plus GPI. | |||
<u>Bivalirudin Angioplasty Trial (BAT)</u> | |||
Angiomax was evaluated in patients with unstable angina undergoing PTCA in two randomized, double-blind, multicenter studies with identical protocols. Patients must have had unstable angina defined as: (1) a new onset of severe or accelerated angina or rest pain within the month prior to study entry or (2) angina or ischemic rest pain which developed between four hours and two weeks after an acute myocardial infarction (MI). Overall, 4312 patients with unstable angina, including 741 (17%) patients with post-MI angina, were treated in a 1:1 randomized fashion with Angiomax or heparin. Patients ranged in age from 29-90 (median 63) years, their weight was a median of 80 kg (39-120 kg), 68% were male, and 91% were Caucasian. Twenty-three percent of patients were treated with heparin within one hour prior to randomization. All patients were administered aspirin 300-325 mg prior to PTCA and daily thereafter. Patients randomized to Angiomax were started on an intravenous infusion of Angiomax (2.5 mg/kg/h). Within 5 min after starting the infusion, and prior to PTCA, a 1 mg/kg loading dose was administered as an intravenous bolus. The infusion was continued for 4 hours, then the infusion was changed under double-blinded conditions to Angiomax (0.2 mg/kg/h) for up to an additional 20 hours (patients received this infusion for an average of 14 hours). The ACT was checked at 5 min and at 45 min following commencement. If on either occasion the ACT was <350 sec, an additional double-blinded bolus of placebo was administered. The Angiomax dose was not titrated to ACT. Median ACT values were: ACT in sec (5th percentile-95th percentile): 345 sec (240-595 sec) at 5 min and 346 sec (range 269-583 sec) at 45 min after initiation of dosing. Patients randomized to heparin were given a loading dose (175 IU/kg) as an intravenous bolus 5 min before the planned procedure, with immediate commencement of an infusion of heparin (15 IU/kg/h). The infusion was continued for 4 hours. After 4 hours of infusion, the heparin infusion was changed under double-blinded conditions to heparin (15 IU/kg/h) for up to 20 additional hours. The ACT was checked at 5 min and at 45 min following commencement. If on either occasion the ACT was <350 sec, an additional double-blind bolus of heparin (60 IU/kg) was administered. Once the target ACT was achieved for heparin patients, no further ACT measurements were performed. All ACTs were determined with the Hemochron® device. The protocol allowed use of open-label heparin at the discretion of the investigator after discontinuation of blinded study medication, whether or not an endpoint event (procedural failure) had occurred. The use of open-label heparin was similar between Angiomax and heparin treatment groups (about 20% in both groups). | |||
The studies were designed to demonstrate the safety and efficacy of Angiomax in patients undergoing PTCA as a treatment for unstable angina as compared with a control group of similar patients receiving heparin during and up to 24 hours after initiation of PTCA. The primary protocol endpoint was a composite endpoint called procedural failure, which included both clinical and angiographic elements measured during hospitalization. The clinical elements were: the occurrence of death, MI, or urgent revascularization, adjudicated under double-blind conditions. The angiographic elements were: impending or abrupt vessel closure. The protocol-specified safety endpoint was major hemorrhage. | |||
The median duration of hospitalization was 4 days for both the Angiomax and the heparin treatment groups. The rates of procedural failure were similar in the Angiomax and heparin treatment groups. Study outcomes are shown in Table 8. | |||
{| | |||
| [[File:Bivalirudin_09.png|thumb|600px]] | |||
|} | |||
<u>AT-BAT Trial</u> | |||
This was a single-group open-label study which enrolled 51 patients with heparin-induced thrombocytopenia (HIT) or heparin induced thrombocytopenia and thrombosis syndrome (HITTS) undergoing PCI. Evidence for the diagnosis of HIT/HITTS was based on a clinical history of a decrease of platelets in patients after heparin administration [new diagnosis or history of clinically suspected or objectively documented HIT/HITTS defined as either: 1) HIT: positive heparin-induced platelet aggregation (HIPA) or other functional assay where the platelet count has decreased to <100,000/mL (minimum 30% from prior to heparin), or has decreased to <150,000/mL (minimum 40% from prior to heparin), or has decreased as above within hours of receiving heparin in a patient with a recent, previous exposure to heparin; 2) HITTS: thrombocytopenia as above plus arterial or venous thrombosis diagnosed by physician examination/laboratory and/or appropriate imaging studies]. Patients ranged in age from 48-89 years (median 70); weight ranged from 42-123 kg (median 76); 50% were male and 50% were female. Angiomax was administered as either 1 mg/kg bolus followed by 2.5 mg/kg/h (high dose in 28 patients) or 0.75 mg/kg bolus followed by a 1.75 mg/kg/h infusion (lower dose in 25 patients) for up to 4 hours. Ninety-eight percent of patients received aspirin, 86% received clopidogrel and 19% received GPIs. | |||
The median ACT values at the time of device activation were 379 sec (high dose) and 317 sec (lower dose). Following the procedure, 48 of the 51 patients (94%) had TIMI grade 3 flow and stenosis <50%. One patient died during a bradycardic episode 46 hours after successful PCI, another patient required surgical revascularization, and one patient experienced no flow requiring a temporary intra-aortic balloon. | |||
Two of the fifty-one patients with the diagnosis of HIT/HITTS developed thrombocytopenia after receiving Angiomax and GPIs.<ref>{{Cite web | last = | first = | title = ANGIOMAX (BIVALIRUDIN) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION [THE MEDICINES COMPANY] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=911cd48f-01ea-4dec-b30c-95e7e0ea9d2a | publisher = | date = | accessdate = 10 March 2014 }}</ref> | |||
==References== | ==References== | ||
Revision as of 18:28, 10 March 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Jesus Rosario Hernandez, M.D. [2]
Clinical Studies
14.1 PCI/PTCA
Angiomax has been evaluated in five randomized, controlled interventional cardiology trials reporting 11,422 patients. Stents were deployed in 6062 of the patients in these trials - mainly in trials performed since 1995. Percutaneous transluminal coronary angioplasty, atherectomy or other procedures were performed in the remaining patients.
REPLACE-2 Trial
This was a randomized, double-blind, multicenter study reporting 6002 (intent-to-treat) patients undergoing PCI. Patients were randomized to treatment with Angiomax with the "provisional" use of platelet glycoprotein IIb/IIIa inhibitor (GPI) or heparin plus planned use of GPI. GPIs were added on a "provisional" basis to patients who were randomized to Angiomax in the following circumstances:
- decreased TIMI flow (0 to 2) or slow reflow;
- dissection with decreased flow;
- new or suspected thrombus;
- persistent residual stenosis;
- distal embolization;
- unplanned stent;
- suboptimal stenting;
- side branch closure;
- abrupt closure; clinical instability; and
- prolonged ischemia.
During the study, one or more of these circumstances occurred in 12.7% of patients in the Angiomax with provisional GPI arm. GPIs were administered to 7.2% of patients in the Angiomax with provisional GPI arm (62.2% of eligible patients).
Patients ranged in age from 25-95 years (median, 63); weight ranged from 35-199 kg (median 85.5); 74.4% were male and 25.6% were female. Indications for PCI included unstable angina (35% of patients), myocardial infarction within 7 days prior to intervention (8% of patients), stable angina (25%) and positive ischemic stress test (24%). Stents were deployed in 85% of patients. Ninety-nine percent of patients received aspirin and 86% received thienopyridines prior to study treatment.
Angiomax was administered as a 0.75 mg/kg bolus followed by a 1.75 mg/kg/h infusion for the duration of the procedure. The activated clotting time (ACT - measured by a Hemochron® device) was measured 5 min after the first bolus of study medication. If the ACT was <225 seconds, an additional bolus of 0.3 mg/kg was given. At investigator discretion, the infusion could be continued following the procedure for up to 4 hours. The median infusion duration was 44 min. Heparin was administered as a 65 U/kg bolus. The activated clotting time (ACT - measured by a Hemochron® device) was measured 5 min after the first bolus of study medication. If the ACT was <225 seconds, an additional bolus of 20 units/kg was given. GPIs (either abciximab or eptifibatide) were given according to manufacturers' instructions. Both randomized groups could be given "provisional" treatments during the PCI at investigator discretion, but under double-blind conditions. "Provisional" treatment with GPI was requested in 5.2% of patients randomized to heparin plus GPI (they were given placebo) and 7.2% patients randomized to Angiomax with provisional GPI (they were given abciximab or eptifibatide according to pre-randomization investigator choice and patient stratification).
The percent of patients reaching protocol-specified levels of anticoagulation was greater in the Angiomax with provisional GPI group than in the heparin plus GPI group. For patients randomized to Angiomax with provisional GPI, the median 5 min ACT was 358 sec (interquartile range 320-400 sec) and the ACT was <225 sec in 3%. For patients randomized to heparin plus GPI, the median 5 min ACT was 317 sec (interquartile range 263-373 sec) and the ACT was <225 sec in 12%. At the end of the procedure, median ACT values were 334 sec (Angiomax group) and 276 sec (heparin plus GPI group).
For the composite endpoint of death, MI, or urgent revascularization adjudicated under double-blind conditions, the frequency was higher (7.6%)(95% confidence interval 6.7%-8.6%) in the Angiomax with "provisional" GPI arm when compared to the heparin plus GPI arm (7.1%)(95% confidence interval 6.1%-8.0%). However, major hemorrhage was reported significantly less frequently in the Angiomax with provisional GPI arm (2.4%) compared to the heparin plus GPI arm (4.1%). Study outcomes are shown in Table 7.
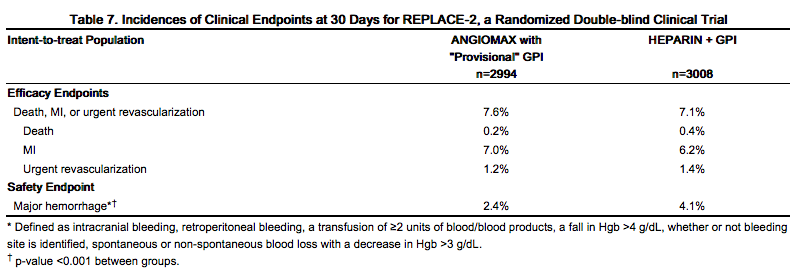 |
At 12 months' follow-up, mortality was 1.9% among patients randomized to Angiomax with "provisional" GPIs and 2.5% among patients randomized to heparin plus GPI.
Bivalirudin Angioplasty Trial (BAT)
Angiomax was evaluated in patients with unstable angina undergoing PTCA in two randomized, double-blind, multicenter studies with identical protocols. Patients must have had unstable angina defined as: (1) a new onset of severe or accelerated angina or rest pain within the month prior to study entry or (2) angina or ischemic rest pain which developed between four hours and two weeks after an acute myocardial infarction (MI). Overall, 4312 patients with unstable angina, including 741 (17%) patients with post-MI angina, were treated in a 1:1 randomized fashion with Angiomax or heparin. Patients ranged in age from 29-90 (median 63) years, their weight was a median of 80 kg (39-120 kg), 68% were male, and 91% were Caucasian. Twenty-three percent of patients were treated with heparin within one hour prior to randomization. All patients were administered aspirin 300-325 mg prior to PTCA and daily thereafter. Patients randomized to Angiomax were started on an intravenous infusion of Angiomax (2.5 mg/kg/h). Within 5 min after starting the infusion, and prior to PTCA, a 1 mg/kg loading dose was administered as an intravenous bolus. The infusion was continued for 4 hours, then the infusion was changed under double-blinded conditions to Angiomax (0.2 mg/kg/h) for up to an additional 20 hours (patients received this infusion for an average of 14 hours). The ACT was checked at 5 min and at 45 min following commencement. If on either occasion the ACT was <350 sec, an additional double-blinded bolus of placebo was administered. The Angiomax dose was not titrated to ACT. Median ACT values were: ACT in sec (5th percentile-95th percentile): 345 sec (240-595 sec) at 5 min and 346 sec (range 269-583 sec) at 45 min after initiation of dosing. Patients randomized to heparin were given a loading dose (175 IU/kg) as an intravenous bolus 5 min before the planned procedure, with immediate commencement of an infusion of heparin (15 IU/kg/h). The infusion was continued for 4 hours. After 4 hours of infusion, the heparin infusion was changed under double-blinded conditions to heparin (15 IU/kg/h) for up to 20 additional hours. The ACT was checked at 5 min and at 45 min following commencement. If on either occasion the ACT was <350 sec, an additional double-blind bolus of heparin (60 IU/kg) was administered. Once the target ACT was achieved for heparin patients, no further ACT measurements were performed. All ACTs were determined with the Hemochron® device. The protocol allowed use of open-label heparin at the discretion of the investigator after discontinuation of blinded study medication, whether or not an endpoint event (procedural failure) had occurred. The use of open-label heparin was similar between Angiomax and heparin treatment groups (about 20% in both groups).
The studies were designed to demonstrate the safety and efficacy of Angiomax in patients undergoing PTCA as a treatment for unstable angina as compared with a control group of similar patients receiving heparin during and up to 24 hours after initiation of PTCA. The primary protocol endpoint was a composite endpoint called procedural failure, which included both clinical and angiographic elements measured during hospitalization. The clinical elements were: the occurrence of death, MI, or urgent revascularization, adjudicated under double-blind conditions. The angiographic elements were: impending or abrupt vessel closure. The protocol-specified safety endpoint was major hemorrhage.
The median duration of hospitalization was 4 days for both the Angiomax and the heparin treatment groups. The rates of procedural failure were similar in the Angiomax and heparin treatment groups. Study outcomes are shown in Table 8.
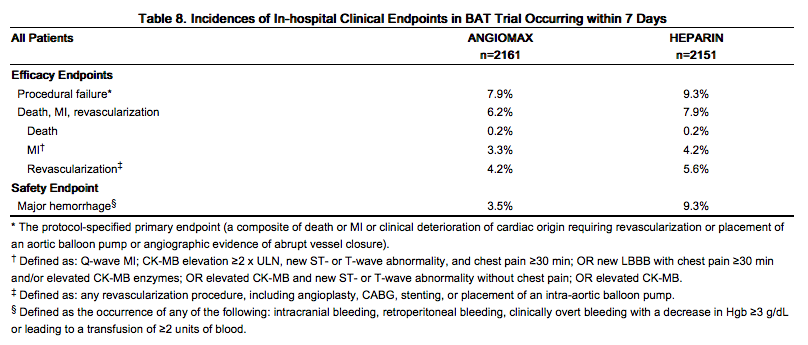 |
AT-BAT Trial
This was a single-group open-label study which enrolled 51 patients with heparin-induced thrombocytopenia (HIT) or heparin induced thrombocytopenia and thrombosis syndrome (HITTS) undergoing PCI. Evidence for the diagnosis of HIT/HITTS was based on a clinical history of a decrease of platelets in patients after heparin administration [new diagnosis or history of clinically suspected or objectively documented HIT/HITTS defined as either: 1) HIT: positive heparin-induced platelet aggregation (HIPA) or other functional assay where the platelet count has decreased to <100,000/mL (minimum 30% from prior to heparin), or has decreased to <150,000/mL (minimum 40% from prior to heparin), or has decreased as above within hours of receiving heparin in a patient with a recent, previous exposure to heparin; 2) HITTS: thrombocytopenia as above plus arterial or venous thrombosis diagnosed by physician examination/laboratory and/or appropriate imaging studies]. Patients ranged in age from 48-89 years (median 70); weight ranged from 42-123 kg (median 76); 50% were male and 50% were female. Angiomax was administered as either 1 mg/kg bolus followed by 2.5 mg/kg/h (high dose in 28 patients) or 0.75 mg/kg bolus followed by a 1.75 mg/kg/h infusion (lower dose in 25 patients) for up to 4 hours. Ninety-eight percent of patients received aspirin, 86% received clopidogrel and 19% received GPIs.
The median ACT values at the time of device activation were 379 sec (high dose) and 317 sec (lower dose). Following the procedure, 48 of the 51 patients (94%) had TIMI grade 3 flow and stenosis <50%. One patient died during a bradycardic episode 46 hours after successful PCI, another patient required surgical revascularization, and one patient experienced no flow requiring a temporary intra-aortic balloon.
Two of the fifty-one patients with the diagnosis of HIT/HITTS developed thrombocytopenia after receiving Angiomax and GPIs.[1]
References
- ↑ "ANGIOMAX (BIVALIRUDIN) INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION [THE MEDICINES COMPANY]". Retrieved 10 March 2014.
Adapted from the FDA Package Insert.