Sumatriptan (oral): Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 58: | Line 58: | ||
'''''For patient information, click [[Sumatriptan oral and nasal (patient information)|here]] and [[Sumatriptan injection (patient information)|here]]'''''. | '''''For patient information, click [[Sumatriptan oral and nasal (patient information)|here]] and [[Sumatriptan injection (patient information)|here]]'''''. | ||
{{SB}} Alsuma<sup>®</sup> | {{SB}} Alsuma<sup>®</sup>, Imitrex<sup>®</sup>, Imitrex STATdose Refill<sup>®</sup>, Imitrex STATdose System<sup>®</sup>, Sumavel DosePro<sup>®</sup> | ||
==Overview== | ==Overview== | ||
| Line 174: | Line 174: | ||
{{Reflist|2}} | {{Reflist|2}} | ||
{{Antimigraine preparations}} | |||
[[Category:Drugs]] | [[Category:Drugs]] | ||
Revision as of 19:45, 3 February 2014
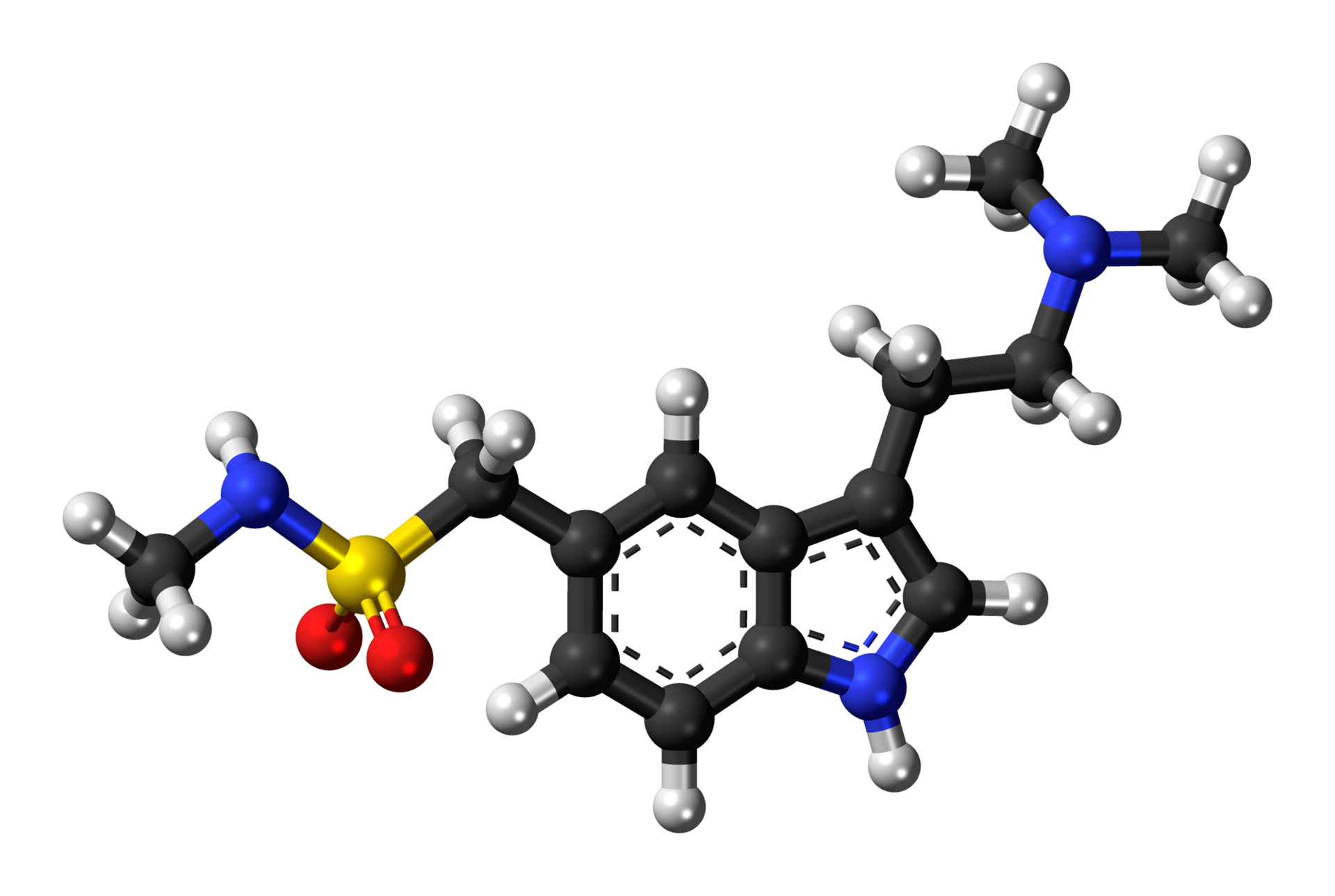 | |
| Clinical data | |
|---|---|
| Trade names | Imitrex, Imigran,Treximet |
| AHFS/Drugs.com | Monograph |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | tablet, subcutaneous injection, nasal spray |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 15% (oral)/ 96% (s.c) |
| Protein binding | 14–21% |
| Metabolism | MAO |
| Elimination half-life | 2.5 hours |
| Excretion | 60% urine; 40% feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H21N3O2S |
| Molar mass | 295.402 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
For patient information, click here and here.
Synonyms / Brand Names: Alsuma®, Imitrex®, Imitrex STATdose Refill®, Imitrex STATdose System®, Sumavel DosePro®
Overview
Sumatriptan is a synthetic drug belonging to the triptan class, used for the treatment of migraine headaches. Structurally, it is an analog of the naturally occurring neuro-active alkaloids dimethyltryptamine (DMT), bufotenine, and 5-methoxy-dimethyltryptamine, with an N-methyl sulfonamidomethyl- group at position C-5 on the indole ring.[1]
Category
Serotonin Receptor Agonists; Antimigraine Agents
FDA Package Insert
IMITREX (sumatriptan succinate) injection
Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
IMITREX (sumatriptan succinate) tablet
Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
IMITREX (sumatriptan) spray
Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
Adverse Effects
Large doses of sumatriptan can cause sulfhemoglobinemia, a rare condition in which the blood changes from red to greenish-black, due to the integration of sulfur into the hemoglobin molecule.[2] If sumatriptan is discontinued, the condition reverses within a few weeks.
Serious cardiac events, including some that have been fatal, have occurred following the use of sumatriptan injection or tablets. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation.[citation needed]
The most common side-effects[3] reported by at least 2% of patients in controlled trials of sumatriptan (25, 50, and 100 mg tablets) for migraine are atypical sensations (paresthesias and warm/cold sensations) reported by 4% in the placebo group and 5–6% in the sumatriptan groups, pain and other pressure sensations (including chest pain) reported by 4% in the placebo group and 6–8% in the sumatriptan groups, neurological events (vertigo) reported by less than 1% in the placebo group and less than 1% to 2% in the sumatriptan groups. Malaise/fatigue occurred in less than 1% of the placebo group and 2–3% of the sumatriptan groups. Sleep disturbance occurred in less than 1% in the placebo group to 2% in the sumatriptan group.
Mechanism of Action
Sumatriptan is structurally similar to serotonin (5-HT), and is a selective agonist for 5-HT1D and 5-HT1B receptors which are present on the cranial vasculature.[4] By activating these receptors, sumatriptan causes vasoconstriction and reduces neurogenic inflammation associated with antidromic neuronal transmission correlating with relief of migraine.
Sumatriptan is also shown to decrease the activity of the trigeminal nerve, which, it is presumed, accounts for sumatriptan's efficacy in treating cluster headaches. The injectable form of the drug has been shown to abort a cluster headache within fifteen minutes in 96% of cases.[5]
Pharmacokinetics
Sumatriptan is administered in several forms; tablets, subcutaneous injection, and nasal spray. Oral administration (as succinate) suffers from poor bioavailability, partly due to presystemic metabolism—some of it gets broken down in the stomach and bloodstream before it reaches the target arteries. A new rapid-release tablet formulation has the same bioavailability, but the maximum concentration is achieved on average 10–15 minutes earlier. When injected, sumatriptan is faster-acting (usually within 10 minutes), but the effect lasts for a shorter time. Sumatriptan is metabolised primarily by monoamine oxidase A into an indole acetic acid analogue, part of which is further conjugated with glucuronic acid. These metabolites are excreted in the urine and bile. Only about 3% of the active drug may be recovered unchanged.
There is no simple, direct relationship between sumatriptan concentration (pharmacokinetics) per se in the blood and its anti-migraine effect (pharmacodynamics). This paradox has, to some extent, been resolved by comparing the rates of absorption of the various sumatriptan formulations, rather than the absolute amounts of drug that they deliver.[6][7]
Approval
Sumatriptan was the first clinically available triptan (in 1991). In the United States, it is available only by medical prescription. However, it can be bought over the counter in the UK and Sweden in 50 mg dosage. Several dosage forms for sumatriptan have been approved, including tablets, solution for injection, and nasal inhalers.
On April 15, 2008, the US FDA approved Treximet, a combination of sumatriptan and naproxen, an NSAID.[8] This combination has shown a benefit over either medicine used separately.[9]
In July 2009, the US FDA approved a single-use jet injector formulation of sumatriptan. The device delivers a subcutaneous injection of 6 mg sumatriptan, without the use of a needle. Autoinjectors with needles have been previously available in Europe and North America for several years.[10]
Phase III studies with a iontophoretic transdermal patch (Zelrix/Zecuity) started in July 2008.[11] This patch uses low voltage controlled by a pre-programmed microchip to deliver a single dose of sumatriptan through the skin within 30 minutes.[12][13] Zecuity was approved by the US FDA in January 2013.[14]
Generics
On November 6, 2008, Par Pharmaceutical announced that it would begin shipping generic versions of sumatriptan injection (sumatriptan succinate injection) 4 mg and 6 mg starter kits and 4 mg and 6 mg pre-filled syringe cartridges to the trade immediately. In addition, Par anticipates launching the 6 mg vials early in 2009.[15]
Mylan Laboratories Inc., Ranbaxy, Sandoz, Dr. Reddy's Pharmaceuticals and other companies have received FDA approval for generic versions of Imitrex tablets in 25-, 50-, and 100-milligram doses since 2009. The drug is available in U.S. and European markets, since Glaxo's patent protections have expired in those jurisdictions. However, sales of a generic delivered via nasal spray are still restricted in the United States.
See also Sumavel DosePro (above).[10]
Chemistry
References
- ↑ The presence of the sulfonamide group in the molecule does not make sumatriptan a "sulfa drug", since it does not have any anti-microbial properties.
- ↑ "Patient bleeds dark green blood". BBC News. 8 June 2007. Retrieved 6 March 2010.
- ↑ Tablets
- ↑ Razzaque Z, Heald MA, Pickard JD; et al. (1999). "Vasoconstriction in human isolated middle meningeal arteries: determining the contribution of 5-HT1B- and 5-HT1F-receptor activation". Br J Clin Pharmacol. 47 (1): 75–82. doi:10.1046/j.1365-2125.1999.00851.x. PMC 2014192. PMID 10073743.
- ↑ Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med 1991;325:322-6.
- ↑ PMID 14756852 (PMID 14756852)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 15953294 (PMID 15953294)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ GSK press release – Treximet (sumatriptan and naproxen sodium) tablets approved by FDA for acute treatment of migraine
- ↑ Brandes JL, Kudrow D, Stark SR; et al. (April 2007). "Sumatriptan-naproxen for acute treatment of migraine: a randomized trial". JAMA. 297 (13): 1443–54. doi:10.1001/jama.297.13.1443. PMID 17405970.
- ↑ 10.0 10.1 PMID 19849720 (PMID 19849720)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Clinical trial number NCT00724815 for "The Efficacy and Tolerability of NP101 Patch in the Treatment of Acute Migraine (NP101-007)" at ClinicalTrials.gov
- ↑ SmartRelief -electronically assisted drug delivery (iontophoresis)
- ↑ Pierce, M; Marbury, T; O'Neill, C; Siegel, S; Du, W; Sebree, T (2009). "Zelrix: a novel transdermal formulation of sumatriptan". Headache. 49 (6): 817–25. doi:10.1111/j.1526-4610.2009.01437.x. PMID 19438727.
- ↑ Zecuity Approved by the FDA for the Acute Treatment of Migraine
- ↑ "PAR PHARMACEUTICAL BEGINS SHIPMENT OF SUMATRIPTAN INJECTION". Par Pharmaceutical. 2008-11-06. Retrieved 2008-11-25.[dead link]
- Pages with script errors
- Pages with non-numeric formatnum arguments
- CS1 maint: Explicit use of et al.
- CS1 maint: Multiple names: authors list
- Pages with incomplete PMID references
- All articles with dead external links
- Articles with dead external links from October 2010
- Articles with invalid date parameter in template
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from December 2011
- Drugs
