Cimetidine (oral): Difference between revisions
No edit summary |
Matt Pijoan (talk | contribs) m (Protected "Cimetidine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
(No difference)
| |
Revision as of 16:40, 27 September 2011
 | |
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, parenteral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60–70% |
| Protein binding | 15–20% |
| Metabolism | Hepatic |
| Elimination half-life | 2 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
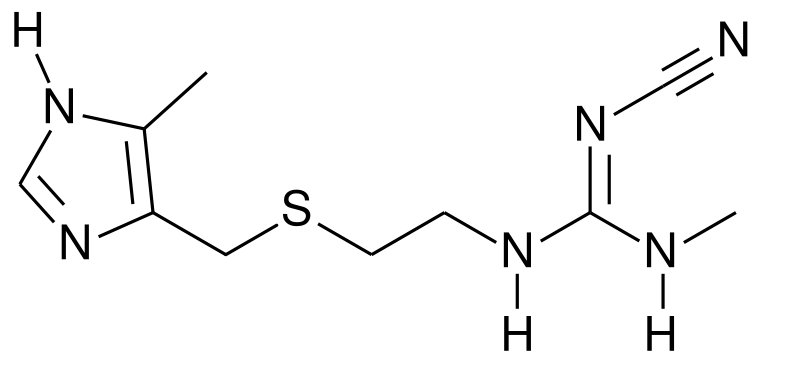
| Formula | C10H16N6S |
| Molar mass | 252.34 g/mol |
|
WikiDoc Resources for Cimetidine (oral) |
|
Articles |
|---|
|
Most recent articles on Cimetidine (oral) Most cited articles on Cimetidine (oral) |
|
Media |
|
Powerpoint slides on Cimetidine (oral) |
|
Evidence Based Medicine |
|
Cochrane Collaboration on Cimetidine (oral) |
|
Clinical Trials |
|
Ongoing Trials on Cimetidine (oral) at Clinical Trials.gov Trial results on Cimetidine (oral) Clinical Trials on Cimetidine (oral) at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cimetidine (oral) NICE Guidance on Cimetidine (oral)
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cimetidine (oral) Discussion groups on Cimetidine (oral) Patient Handouts on Cimetidine (oral) Directions to Hospitals Treating Cimetidine (oral) Risk calculators and risk factors for Cimetidine (oral)
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cimetidine (oral) |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Cimetidine (INN) (Template:PronEng) is a histamine H2-receptor antagonist that inhibits the production of acid in the stomach. It is largely used in the treatment of heartburn and peptic ulcers. It is marketed by GlaxoSmithKline under the trade name Tagamet (sometimes Tagamet HB or Tagamet HB200) and was approved by the Food & Drug Administration for prescriptions starting January 1, 1979.
Clinical use
Main article:H2-receptor antagonist
History and development
Cimetidine was the prototypical histamine H2-receptor antagonist from which the later members of the class were developed. Cimetidine was the culmination of a project at Smith, Kline & French (SK&F; now GlaxoSmithKline) to develop a histamine receptor antagonist to suppress stomach acid secretion.
At the time (1964) it was known that histamine was able to stimulate the secretion of stomach acid, but also that traditional antihistamines had no effect on acid production. In the process, the SK&F scientists also proved the existence of histamine H2-receptors.
The SK&F team used a rational drug-design structure starting from the structure of histamine - the only design lead, since nothing was known of the then hypothetical H2-receptor. Hundreds of modified compounds were synthesised in an effort to develop a model of the receptor. The first breakthrough was Nα-guanylhistamine, a partial H2-receptor antagonist. From this lead the receptor model was further refined and eventually led to the development of burimamide, the first H2-receptor antagonist. Burimamide, a specific competitive antagonist at the H2-receptor 100-times more potent than Nα-guanylhistamine, proved the existence of the H2-receptor.
Burimamide was still insufficiently potent for oral administration and further modification of the structure, based on modifying the pKa of the compound, lead to the development of metiamide. Metiamide was an effective agent, however it was associated with unacceptable nephrotoxicity and agranulocytosis. It was proposed that the toxicity arose from the thiourea group, and similar guanidine-analogues were investigated until the ultimate discovery of cimetidine.
Other uses
There have been two studies relating to the use of Cimetidine for treatment of warts in children. According to the studies, a daily dosage of 400mg of Cimetidine can remove over 200 warts from a 15 year old child.[2]
Another study by Yokoyama et al used Cimetidine for the treatment of Chronic Calcifying Tendonitis of the shoulder. The small scale study took 16 individuals with calcifying tendonitis in one shoulder, all of which had previously attempted other forms of therapy including steroid injection and arthroscopic lavage. During the course of the study 10 patients reported an elimination of pain and 9 displayed a complete disappearance of Calcium deposits. With results being on a small scale, it has been recommended that Cimetidine, for the treatment of chronic calcifying tendonitis of the shoulder, be opened to large scale clinical trials. [3]
Cimetidine has also been reported for use in treatment of colorectal cancer - it is however not approved in the US by the FDA for cancer treatment.
Shortcomings and side effects
Cimetidine is a known inhibitor of many isozymes of the cytochrome P450 enzyme system (specifically CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4). This inhibition forms the basis of the numerous drug interactions that occur between cimetidine and other drugs. For example, cimetidine may decrease metabolism of some drugs, such as those used in hormonal contraception. Cimetidine interferes with metabolism of the hormone estrogen, enhancing estrogen activity. In women, this can lead to galactorrhea, whereas in men gynecomastia and a reduced sperm count can result. Adverse drug reactions were also found to be relatively common with Cimetidine, including interactions with the antimalarial medication Hydroxychloroquine.
The development of longer-acting H2-receptor antagonists with reduced adverse effects such as ranitidine proved to be the downfall of cimetidine and, whilst it is still used, it is no longer amongst the more widely used H2-receptor antagonists. Cimetidine should be used with caution is causes of hepatic impairment and cardiovascular disease. Side effects can include dizziness, and more rarely, headache. BIOAVAILABILITY: The observed bioavailibility of cimetidine is almost 60%.
References
- Michnovicz JJ, Galbraith RA .Cimetidine inhibits catechol estrogen metabolism in women. Metabolism. 1991 Feb;40(2):170-4. PMID 1988774
Template:H2-receptor antagonist
de:Cimetidin hr:Cimeditin nl:Cimetidine fi:Simetidiini th:ไซเมติดีน
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Guanidines
- H2 receptor antagonists
- Imidazoles
- Thioethers
- Drugs