Human ehrlichiosis: Difference between revisions
No edit summary |
(No difference)
|
Revision as of 14:35, 6 January 2009
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
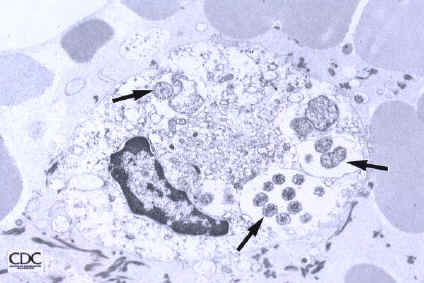
Toward the end of the 19th century, scientists began to understand the important potential for ticks to act as transmitters of disease. In the last decades of the 20th century, several tick-borne diseases have been recognized in the United States, including babesiosis, Lyme disease, and ehrlichiosis.
Ehrlichiosis is caused by several bacterial species in the genus Ehrlichia (pronounced err-lick-ee-uh) which have been recognized since 1935. Over several decades, veterinary pathogens that caused disease in dogs, cattle, sheep, goats, and horses were identified. Currently, three species of Ehrlichia in the United States and one in Japan are known to cause disease in humans; others could be recognized in the future as methods of detection improve.
In 1953, the first ehrlichial pathogen of humans was identified in Japan. Sennetsu fever, caused by Ehrlichia sennetsu, is characterized by fever and swollen lymph nodes. The disease is very rare outside the Far East and Southeast Asia, and most cases have been reported from western Japan.
In the United States, human diseases caused by Ehrlichia species have been recognized since the mid-1980s. The ehrlichioses represent a group of clinically similar, yet epidemiologically and etiologically distinct, diseases caused by Ehrlichia chaffeensis, E. ewingii, and a bacterium extremely similar or identical to E. phagocytophila. The remainder of the information on this web page will focus on the types of ehrlichiosis that occur in the United States.
Human ehrlichiosis due to Ehrlichia chaffeensis was first described in 1987. The disease occurs primarily in the southeastern and south central regions of the country and is primarily transmitted by the lone star tick, Amblyomma americanum.
Human granulocytic ehrlichiosis (HGE) represents the second recognized ehrlichial infection of humans in the United States, and was first described in 1994. The name for the species that causes HGE has not been formally proposed, but this species is closely related or identical to the veterinary pathogens Ehrlichia equi and Ehrlichia phagocytophila. HGE is transmitted by the blacklegged tick (Ixodes scapularis) and the western blacklegged tick (Ixodes pacificus) in the United States.
Ehrlichia ewingii is the most recently recognized human pathogen. Disease caused by E. ewingii has been limited to a few patients in Missouri, Oklahoma, and Tennessee, most of whom have had underlying immunosuppression. The full extent of the geographic range of this species, its vectors, and its role in human disease is currently under investigation.
Related Key Words and Synonyms:
Epidemiology and Demographics
United States
During 1986 to 1997, health departments and other diagnostic laboratories reported over 1200 cases of human ehrlichiosis to CDC. Approximately two-thirds were cases of HME. CDC compiles the number of cases reported by the state health departments. Ehrlichiosis is a nationally notifiable disease; however, not all state health departments have reported cases of ehrlichiosis to CDC (Figure 1). To ensure standardization across the country, a consistent case definition is used by all states when reporting cases to CDC.
- Figure 1: Reported Cases of Ehrlichiosis in the United States

The occurrence of these diseases mirrors the geographic distributions and seasonal activities of the tick vectors. Most patients with ehrlichiosis are infected in the spring and summer when they are more commonly exposed to vector ticks. Accordingly, 80% to 90% of all ehrlichiosis cases occur between April and September, and approximately 55% to 70% of all cases occur during May through July (Figure 2). This period is the season for adult Amblyomma americanum and nymphal Ixodes scapularis ticks. A history of tick bite or exposure to tick-infested habitats is reported in 50% to 90% of cases.
- Figure 2: Approximate seasonal distribution of HGE in the United States

Ehrlichia chaffeensis infections are most frequently reported from southeastern and midwestern states with abundant lone star tick populations, especially Arkansas, Florida, Georgia, Missouri, North Carolina, Oklahoma, Tennessee, Texas, and Virginia. Cases have been reported from almost every state in the United States, although some of these may have been imported from states where the disease is highly endemic.
The types of ehrlichiosis are distinct from several other well-described tick-transmitted diseases in the United States with respect to age-specific incidence (Figure 3). In general, reported rates of ehrlichiosis increase with age; most patients with disease appear to be older adults (most often >40 years old). This pattern contrasts with age-specific incidences of Lyme disease and Rocky Mountain spotted fever, which occur most frequently in children. Age-associated host factors may account for severity of disease; however, severe and even fatal ehrlichial infections have occurred in otherwise healthy young adults and children.
- Figure 3: Average annual reported HGE rate (per 100,000) by age group, in NY and CT, 1995-1997

Most of the recognized HGE cases have originated from states that also have a high incidence of Lyme disease, particularly Connecticut, Minnesota, New York, and Wisconsin. This distribution is consistent with the fact that the vector of HGE, Ixodes scapularis, also transmits Borrelia burgdorferi. A number of other states have also reported HGE cases. In the western United States, the HGE agent is transmitted by the western blacklegged tick, Ixodes pacificus (Figure 4).
- Figure 4: Areas where human ehrlichiosis may occur based on approximate distribution of vector tick species

Relatively few population-based investigations have pursued the fundamental question of how many persons become ill after infection with ehrlichiae. A study conducted in southeastern Georgia during 1987-1988 examined the incidence of E. chaffeensis infection among hospitalized patients with fever and determined an incidence of 5.3 cases per 100,000 total population per year. Passive surveillance data for these infections are also sparse, and are collected from relatively few regions of the United States where Ehrlichia species are endemic.
As of 1999, cases of ehrlichiosis must be reported to the state health department in at least 20 states (Alabama, Arkansas, Arizona, California, Connecticut, Delaware, Florida, Georgia, Kentucky, Maine, Michigan, Minnesota, Missouri, Montana, New Jersey, New Hampshire, New York, North Carolina, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, and Utah) (Figure 5).
- Figure 5: States where Ehrlichiosis is a notifiable disease

Data from statewide laboratory-based surveillance initiated by New York in 1994 and by Connecticut in 1995 revealed annual reporting rates of ehrlichiosis of 0.4 and 1.8 cases per 100,000 persons, respectively. Foci of disease have been identified in defined geographical regions with reported annual rates as high as 16 cases per 100,000 persons for HGE and 31 cases per 100,000 persons for HME infection.
Worldwide
Ehrlichial pathogens are distributed globally, primarily in temperate regions. Patients with serologic evidence of infection with E. chaffeensis or, more likely, with a species antigenically related to E. chaffeensis have been identified in several other countries, including Argentina, Belgium, Israel, Italy, Mali, Mexico, Portugal, and Thailand. Similarly, human infections with E. phagocytophila have been confirmed in Belgium, Denmark, Hungary, Slovenia, and Sweden, and persons with antibodies reactive to granulocytic ehrlichiae have been identified in Germany, Israel, Italy, Norway, Switzerland, and the United Kingdom.
Pathophysiology & Etiology
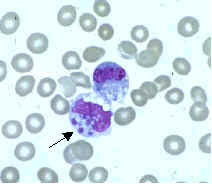
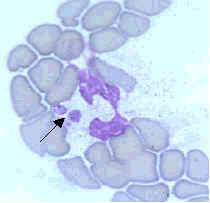
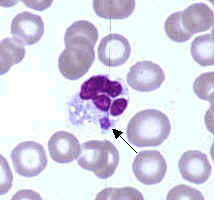
Ehrlichiae are small, gram-negative bacteria that primarily invade leukocytes (white blood cells), the same cells which fight disease by destroying microorganisms that enter the body. Ehrlichiae typically appear as minute, round bacteria (cocci), ranging from 1 to 3 µm (micrometers) in diameter. In the leukocytes, ehrlichiae divide to form vacuole-bound colonies known as morulae (plural for morula, which is the Latin word for mulberry, referring to the mulberry-like clustering of the dividing organisms). The formation of morulae is a defining characteristic of this group of bacterial pathogens (Figure A).
Taxonomy:
The genus Ehrlichia is currently classified as a member of the tribe Ehrlichieae, of the family Rickettsiaceae, in the order Rickettsiales. The genus includes seven recognized species: E. canis, E. chaffeensis, E. equi, E. phagocytophila, E. risticii, E. ewingii, and E. sennetsu. A number of other named ehrlichiae, such as "E. platys," "E. bovis," E. ovina," and "E. ondiri," also cause disease in animals (Table 1). The names of the latter organisms are enclosed in quotation marks because they have not been formally proposed and accepted according to the rules of the International Code of Nomenclature of Bacteria, Bacteriological Code.
The ehrlichiae were initially grouped according to the type of blood cell most commonly infected (granulocyte, lymphocyte, monocyte, platelet), and disease classes have been termed "granulocytic (or granulocytotropic) ehrlichiosis" or "monocytic (or monocytotropic) ehrlichiosis." However, this type of classification may be misleading because some of the Ehrlichia species have been found in cells other than their chief target cell type. In addition, more than one species may be responsible for the broad category of "monocytic" or "granulocytic" ehrlichiosis (e.g., compare the HGE agent and E. ewingii in the figures below).
- Figure B. Ehrlichia chaffeensis primarily infects mononuclear leukocytes (predominantly monocytes and macrophages), but may also be seen occasionally in the granulocytes of some patients with severe disease.
- Figure C. The pathogen that causes human granulocytic ehrlichiosis (HGE) primarily infects granulocytes (neutrophils and rarely eosinophils). The pathogen is often referred to as the agent of HGE or the HGE agent. This species is very similar, or likely identical, to E. phagocytophila and E. equi.
- Figure D. Ehrlichia ewingii primarily infects neutrophils and occasionally eosinophils and produces a disease clinically similar to HME and HGE. Most patients with this form of ehrlichiosis have also had other medical conditions causing immunosuppression (e.g., HIV infection, splenectomy, transplantation, immunosuppressive drugs).
-
Figure B
-
Figure C
-
Figure D
Modern Classification:
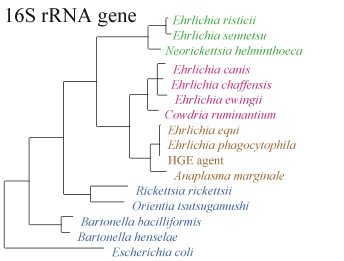
Using modern molecular biology techniques, we now know that Ehrlichia species form three distinct groups (see Figure 5 below). The species contained within these "genogroups" are also related to organisms not previously considered to be members of this genus. The classification of the genus Ehrlichia requires revision, and future studies may provide the additional data needed.
- Figure D. Genetic relationship of Ehrlichia species and other bacteria based on similarity of 16rRNA gene
Natural History
Studies throughout the world have shown that many cases of human illness are caused by zoonotic pathogens that are maintained by animal hosts in their natural cycles. Many zoonoses require a vector (e.g., mosquito, tick, mite) to be transmitted from the animal host to the human host. The ehrlichial disease cycle includes four components: the pathogen, the animal reservoir species, the vectors, and the human host. For the types of human ehrlichiosis in the United States, the arthropod vectors are ticks.
Only one of the three tick families, Ixodidae (hard ticks), is associated with ehrlichiae. These ticks have four stages in their life cycle: egg, larva, nymph, and adult. After the eggs hatch, each stage must feed once to develop into the next stage. Larvae are uninfected with ehrlichiae when they begin to look for a bloodmeal. Ticks become infected with ehrlichiae while feeding on blood from the host in either the larval or nymphal stage. After the tick develops into the next stage, the ehrlichiae may be transmitted to the following host during the feeding process. Both male and female ticks may bite humans but it is the females that are responsible for most transmission. In the United States, it appears that both the nymphal and adult stages are responsible for transmission of ehrlichiae, but one stage may be more important for each Ehrlichia species.
Diagnosis
A diagnosis of ehrlichiosis is based on a combination of clinical signs and symptoms and confirmatory laboratory tests. Your doctor can send your blood sample to a reference laboratory for testing. However, the availability of the different types of laboratory tests varies considerably. Other laboratory findings indicative of ehrlichiosis include low white blood cell count, low platelet count, and elevated liver enzymes.
History and Symptoms
The early clinical presentations of ehrlichiosis may resemble nonspecific signs and symptoms of various other infectious and non-infectious diseases. It is unclear if all persons infected with ehrlichiae become ill. It is possible that many infected persons develop an illness so mild they do not seek medical attention or perhaps have no symptoms at all.
Patients with ehrlichiosis generally visit a physician in their first week of illness, following an incubation period of about 5-10 days after the tick bite. Initial symptoms generally include fever, headache, malaise, and myalgia. Other signs and symptoms may include nausea, vomiting, diarrhea, cough, joint pains, confusion, and occasionally rash. In contrast to Rocky Mountain spotted fever, rash is relatively uncommon in adult patients with HME, and is rarely reported with HGE. However, rash has been described in approximately 60% of pediatric patients infected with E. chaffeensis.
Laboratory findings indicative of ehrlichiosis include leukopenia, thrombocytopenia, and elevated liver enzymes. See Laboratory Detection for more information on laboratory confirmation of ehrlichiosis.
Ehrlichiosis can be a severe illness, especially if untreated, and as many as half of all patients require hospitalization. Severe manifestations of the disease may include prolonged fever, renal failure, disseminated intravascular coagulopathy, meningoencephalitis, adult respiratory distress syndrome, seizures, or coma. It is estimated that 2%-3% of patients may die from the infection. Preliminary evidence suggests that E. chaffeensis infection may become more severe than other ehrlichial infections.
The severity of ehrlichiosis may be related in part to the immune status of the patient. Persons with compromised immunity caused by immunosuppresive therapies (e.g., cortiocosteroids or cancer chemotherapy), HIV infection, or splenectomy appear to develop more severe disease, and case-fatality ratios for these individuals are characteristically higher than case-fatality ratios reported for the general population.
Laboratory Findings

Ehrlichial infections pose difficult diagnostic challenges to both clinicians and laboratorians, and the availability of confirmatory assays is limited. Therefore, treatment decisions should be based on epidemiologic and clinical clues, and should never be delayed while waiting for confirmation. Similarly, test results should be interpreted in the context of the patient’s illness and the epidemiologic setting. Problems arise from overuse of specialized tests for patients with a low probability of the disease and in areas with a low prevalence of disease. Fundamental understanding of the signs, symptoms, and epidemiology of the disease is crucial in guiding requests for tests for ehrlichiosis and interpretation of testing results. Routine clinical laboratory tests indicative of ehrlichiosis include low white blood cell count, low platelet count, and elevated liver enzymes. The organisms can be demonstrated in blood smears by staining with Diff-Quik or Giemsa stains (Figure 7).
Laboratory confirmation of ehrlichiosis requires serologic, molecular, or culture-based methods. Serologic evaluations are conducted by using the indirect immunofluorescence assay (IFA). Antibodies in the serum bind to the organisms on a slide and are detected by a fluorescein-labeled conjugate (Figure 6). Although IFA remains the principal diagnostic tool for the detection of ehrlichial infection, there is no standardized antigen, conjugate, or agreement on what constitutes a positive result among the various laboratories providing these tests. Individual laboratories should be consulted as to their test threshold levels. Blood specimens taken early (acute) and late (convalescent) in the disease course represent the preferred specimens for evaluation. Relatively few data describe the kinetics of IFA-detectable antibodies for the types of ehrlichiosis. Most patients demonstrate increased IgM or IgG titers by the second week of the illness. However, patients may lack diagnostic IgG antibody titers in the first 7 days of illness. This is an important consideration, because patients seek health care at a median of 3½ days after onset of the illness. The period for which anti-ehrlichial antibodies persist is also poorly defined. In some persons, high titers have been observed for longer than 2½ years after the acute illness. Most reference laboratories conduct testing for IgG; however, IgM-specific testing may provide helpful evidence of ehrlichial infection.
Antibodies reactive with one Ehrlichia species can be cross-reactive with other species of Ehrlichia. The diagnosis of HGE is based on seroreactivity to any of several strains of granulocytic ehrlichiae. However, serologic cross-reactivity between different genogroups is also observed and may hamper epidemiologic distinction between the ehrlichial infections. It is possible that some serologically confirmed cases of infection thought to be caused by E. chaffeensis or the HGE agent may in fact represent infections with the other agent or with another, antigenically related ehrlichial species. Although cross-reaction with E. canis antigen was used to identify the initial cases of E. chaffeensis infection, this antigen is no longer appropriate for serodiagnosis.

After serologic methods, amplification of the ehrlichial DNA by polymerase chain reaction (PCR) is the next most frequently used method for detecting infection. This test is available through CDC and some state health laboratories, as well as a number of research and commercial laboratories. PCR tests remain unstandardized, and analytical and diagnostic sensitivity and specificity may vary among individual assays. In HGE, the organism has been detected by PCR from the blood of clinically ill patients 3-5 weeks following the onset of symptoms. In persons infected with E. chaffeensis, ehrlichial DNA has been detected by PCR from febrile, untreated patients as long as 7 weeks into the illness.
Direct isolation of the organism remains the gold standard for confirmatory diagnosis, but is the most difficult and time-consuming approach. Both E. chaffeensis and the HGE agent have been recovered from the blood of acutely ill patients by using a variety of cell lines, most frequently canine DH82 (Figure 7) and human HL-60 cells, respectively. Ehrlichia chaffeensis has typically been observed within 7-36 days in culture; the HGE agent may be seen within 7-12 days after inoculation of cells with patient blood. EDTA-treated blood obtained prior to the administration of antibiotic therapy is the most common specimen for isolation attempts, as well as the most frequent sample used for PCR analysis. Samples should be processed as quickly as possible, although successful recovery of viable ehrlichiae may result from samples refrigerated at 4oC for as long as 1 week.
New techniques, including enzyme immunoassays using recombinant ehrlichial antigens and fluorometric PCR, are currently under investigation, and these tests may eventually have broader application in public health laboratories.
Risk Stratification and Prognosis
Can a person get ehrlichiosis more than once?
Very little is known about immunity to ehrlichial infections. Although it has been proposed that infection with ehrlichiae confers long-term protection against reinfection, there have been occassional reports of laboratory-confirmed reinfection. Short-term protection has been described in animals infected with some Ehrlichia species and this protection wanes after about 1 year. Clearly, more studies are needed to determine the extent and duration of protection against reinfection in humans.
Treatment
Appropriate antibiotic treatment should be initiated immediately when there is a strong suspicion of ehrlichiosis on the basis of clinical and epidemiologic findings. Treatment should not be delayed until laboratory confirmation is obtained. Fever generally subsides within 24-72 hours after treatment with doxycycline or other tetracyclines. In fact, failure to rapidly respond to a tetracycline antibiotic argues against a diagnosis of ehrlichiosis. Preventative therapy in non-ill patients who have had recent tick bites is not warranted.
Acute Pharmacotherapies
Doxycycline (100 mg twice daily for adults or 4.4 mg/kg body weight per day in two divided doses for children under 45.4 kgs (100 lbs)) is the drug of choice for patients with ehrlichiosis. The optimal duration of therapy has not been established, but current regimens recommend continuation of treatment for at least 3 days after the fever subsides and until evidence of clinical improvement, for a minimum total course of 5 to 7 days. Severe or complicated disease may require longer treatment courses. Because tetracyclines are contraindicated in pregnancy, rifampin has been used successfully in a limited number of pregnant women with documented HGE.
Primary Prevention
Limiting exposure to ticks reduces the likelihood of ehrlichial infection. In persons exposed to tick-infested habitats, prompt careful inspection and removal of crawling or attached ticks is an important method of preventing disease. It may take several hours of attachment before microorganisms are transmitted from the tick to the host.
It is unreasonable to assume that a person can completely eliminate activities that may result in tick exposure. Therefore, prevention measures should be aimed at personal protection:
- Wear light-colored clothing -- this will allow you to see ticks that are crawling on your clothing.
- Tuck your pants legs into your socks so that ticks cannot crawl up the inside of your pants legs.
- Apply repellants to discourage tick attachment. Repellents containing permethrin can be sprayed on boots and clothing, and will last for several days. Repellents containing DEET (n, n-diethyl-m-toluamide) can be applied to the skin, but will last only a few hours before reapplication is necessary. Use DEET with caution on children because adverse reactions have been reported.
- Conduct a body check upon return from potentially tick-infested areas by searching your entire body for ticks. Use a hand-held or full-length mirror to view all parts of your body. Remove any tick you find on your body.
To remove attached ticks, use the following procedure:
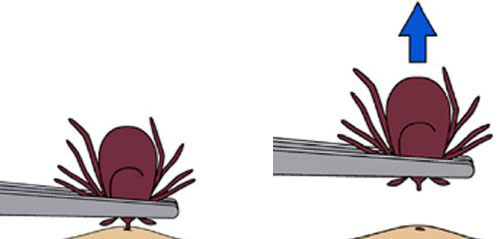
- Use fine-tipped tweezers or shield your fingers with a tissue, paper towel, or rubber gloves.
- Grasp the tick as close to the skin surface as possible and pull upward with steady, even pressure. Do not twist or jerk the tick; this may cause the mouthparts to break off and remain in the skin. (If this happens, remove mouthparts with tweezers. Consult your healthcare provider if infection occurs.)
- Do not squeeze, crush, or puncture the body of the tick because its fluids (saliva, hemolymph, gut contents) may contain infectious organisms.
- Do not handle the tick with bare hands because infectious agents may enter through mucous membranes or breaks in the skin. This precaution is particularly directed to individuals who remove ticks from domestic animals with unprotected fingers. Children, elderly persons, and immunocompromised persons may be at greater risk of infection and should avoid this procedure.
- After removing the tick, thoroughly disinfect the bite site and wash your hands with soap and water.
- You may wish to save the tick for identification in case you become ill within 2 to 3 weeks. Your doctor can use the information to assist in making an accurate diagnosis. Place the tick in a plastic bag and put it in your freezer. Write the date of the bite on a piece of paper with a pencil and place it in the bag.
Note: Folklore remedies such as petroleum jelly or hot matches do little to encourage a tick to detach from skin. In fact, they may make matters worse by irritating the tick and stimulating it to release additional saliva, increasing the chances of transmitting the pathogen. These methods of tick removal should be avoided. In addition, a number of tick removal devices have been marketed, but none are better than a plain set of fine tipped tweezers.
Secondary Prevention
Tick Control:
Strategies to reduce vector tick densities through area-wide application of acaricides (chemicals that will kill ticks and mites) and control of tick habitats (e.g., leaf litter and brush) have been effective in small-scale trials. New methods under development include applying acaricides to rodents and deer by using baited tubes, boxes, and deer feeding stations in areas where these pathogens are endemic. Biological control with fungi, parasitic nematodes, and parasitic wasps may play important roles in integrated tick control efforts. Community-based integrated tick management strategies may prove to be an effective public health response to reduce the incidence of tick-borne infections. However, limiting exposure to ticks is presently the most effective method of prevention.
References
- http://www.cdc.gov/ncidod/dvrd/ehrlichia/Index.htm
- http://www.cdc.gov/ncidod/dvrd/ehrlichia/Natural_Hx/Natural_Hx.htm
- http://www.cdc.gov/ncidod/dvrd/ehrlichia/Prevention/Prevention.htm
- http://www.cdc.gov/ncidod/dvrd/ehrlichia/Q&A/Q&A.htm
- http://www.cdc.gov/ncidod/dvrd/ehrlichia/Organisms/Organism.htm
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.