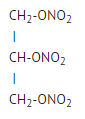Nitroglycerin (Topical ointment): Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
<!--Overview--> | <!--Overview--> | ||
|genericName= | |genericName=nitroglycerin (ointment) | ||
| Line 23: | Line 23: | ||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
|adverseReactions= | |adverseReactions= | ||
| Line 44: | Line 44: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
*RECTIV™ (nitroglycerin) Ointment 0.4% is indicated for the treatment of moderate to severe pain associated with chronic anal fissure. | |||
=====Dosage===== | |||
*Apply 1 inch of ointment (375 mg of ointment equivalent to 1.5 mg of nitroglycerin) intra-anally every 12 hours for up to 3 weeks. A finger covering, such as plastic-wrap, disposable surgical glove or a finger cot, should be placed on the finger to apply the ointment. To obtain a 1.5 mg dose of nitroglycerin, the covered finger is laid alongside the 1 inch dosing line on the carton. | |||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Refer to carton for accurate dosage guide. | |||
*The tube is gently squeezed until a line of ointment the length of the measuring line is expressed onto the covered finger. The ointment is gently inserted into the anal canal using the covered finger no further than to the first finger joint and the ointment is applied around the side of the anal canal. If this cannot be achieved due to pain, application of the ointment should be made directly to the outside of the anus. Treatment may be continued for up to three weeks. | |||
*RECTIV ointment is not for oral, ophthalmic, or intravaginal use. Hands should be washed after application of the ointment. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 78: | Line 84: | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
=====PDE5 inhibitor use===== | |||
*Administration of RECTIV is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), such as sildenafil, vardenafil, and tadalafil, as these are shown to potentiate the hypotensive effects of organic nitrates [see 7.1 DRUG INTERACTIONS]. | |||
===== | =====Severe anemia | ||
===== | |||
*RECTIV is contraindicated in patients with severe anemia. | |||
===== | =====Increased intracranial pressure | ||
===== | |||
*RECTIV is contraindicated in patients with increased intracranial pressure. | |||
=====Hypersensitivity | |||
===== | |||
*RECTIV is contraindicated in patients who have shown hypersensitivity to it or to other nitrates or nitrites. Skin reactions consistent with hypersensitivity have been observed with organic nitrates. | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings= | ||
* | =====Cardiovascular disorders===== | ||
*Venous and arterial dilatation as a consequence of nitroglycerin treatment including RECTIV, can decrease venous blood returning to the heart and reduce arterial vascular resistance and systolic pressure. Exercise caution when treating patients with any of the following conditions: blood volume depletion, existing hypotension, cardiomyopathies, congestive heart failure, acute myocardial infarction, or poor cardiac function for other reasons. If patients with any of these conditions are treated with RECTIV, monitor cardiovascular status and clinical condition. The adverse reactions of RECTIV are likely to be more pronounced in the elderly. | |||
==== | =====Headache===== | ||
* | *RECTIV produces dose-related headaches, which may be severe. Tolerance to headaches occurs. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 129: | Line 137: | ||
|clinicalTrials= | |clinicalTrials= | ||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
* | *The most common adverse reaction of RECTIV (nitroglycerin) Ointment 0.4% applied to the anal canal is headache. | ||
* | *Headache may be recurrent following each dose. Headaches are typically of short duration and can be treated with an analgesic, e.g. acetaminophen, and are reversible upon discontinuation of treatment. | ||
* | *In Study REC-C-001, a double-blind, placebo-controlled trial in patients with a painful chronic anal fissure, the most frequent (≥ 2%) adverse reactions reported were as follows (Table 1): | ||
* | : [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
=====Hypotension===== | |||
*Transient episodes of light-headedness, occasionally related to blood pressure changes, also may occur. Hypotension (including orthostatic hypotension) occurs infrequently, but in some patients may be severe enough to warrant discontinuation of therapy. | |||
=====Allergic Reactions===== | |||
*Flushing, allergic reactions and application site reactions (including drug rash and exfoliative dermatitis) have been reported rarely. | |||
=====Methemoglobinemia===== | |||
*In rare cases, therapeutic doses of organic nitrates have caused methemoglobinemia | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing= | |postmarketing= | ||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
| Line 148: | Line 164: | ||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
=====PDE5 inhibitors===== | |||
*Phosphodiesterase type 5 (PDE5) inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. | |||
*The time course of the interaction appears to be related to the half-life of the PDE5 inhibitor, however, the dose dependence of this interaction has not been studied. Use of RECTIV within a few days of PDE5 inhibitors is contraindicated. | |||
=====Antihypertensives===== | |||
*Patients receiving antihypertensive drugs, beta-adrenergic blockers, and other nitrates should be observed for possible additive hypotensive effects when using RECTIV. Marked orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used concomitantly. | |||
*Beta-blockers blunt the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effects. If beta-blockers are used with RECTIV in patients with angina pectoris, additional hypotensive effects may occur. | |||
=====Aspirin===== | |||
*Coadministration of aspirin (at doses between 500 mg and 1000 mg) and nitroglycerin has been reported to result in increased nitroglycerin maximum concentrations by as much as 67% and AUC by 73% when administered as a single dose. The pharmacological effects of RECTIV may be enhanced by concomitant administration of aspirin. | |||
=====Tissue-type Plasminogen Activator (t-PA)===== | |||
*Intravenous administration of nitroglycerin decreases the thrombolytic effect of tissue-type plasminogen activator (t-PA). Plasma levels of t-PA are reduced when coadministered with nitroglycerin. Therefore, caution should be observed in patients receiving RECTIV during t-PA therapy. | |||
===== | =====Heparin===== | ||
*Although an interaction has been reported between intravenous heparin and intravenous nitroglycerin (resulting in a decrease in the anticoagulant effect of heparin), the data are not consistent. If patients are to receive intravenous heparin and RECTIV concurrently, the anticoagulation status of the patient must be checked. | |||
=====Ergotamine===== | |||
*Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and consequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore the possibility of ergotism in patients receiving RECTIV should be considered. | |||
=====Alcohol===== | |||
*The vasodilating effects of nitroglycerin have been shown to be additive to the effects observed with alcohol.<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
*Pregnancy Category CAnimal reproduction and teratogenicity studies have not been conducted with RECTIV. Nitroglycerin was not teratogenic when administered by topical or dietary route. There are no adequate and well-controlled studies in pregnant women. RECTIV should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
*Teratology studies in rats and rabbits were conducted with topically applied nitroglycerin ointment at doses up to 80 mg/kg/day and 240 mg/kg/day, respectively. No toxic effects on dams or fetuses were seen at any dose tested. | |||
* | *A teratogenicity study was conducted in rats with nitroglycerin administered in the diet at levels up to 1% content (approximately 430 mg/kg/day) on days 6 to 15 of gestation. In offspring of the high-dose group, an increased but not statistically significant incidence of diaphragmatic hernias was noted together with decreased hyoid bone ossification. The latter finding probably reflects delayed development, thus indicating no clear evidence of a potential teratogenic effect of nitroglycerin. | ||
|useInPregnancyAUS= | |||
| | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
| Line 197: | Line 218: | ||
|useInNursing= | |useInNursing= | ||
*It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when RECTIV is administered to a nursing woman. | |||
|useInPed= | |useInPed= | ||
*The safety and effectiveness of RECTIV in pediatric patients under 18 years of age have not been established. | |||
|useInGeri= | |useInGeri= | ||
*Clinical studies of RECTIV did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Clinical data from the published literature indicate that the elderly demonstrate increased sensitivity to nitrates, which may be therapeutic but also manifest by more frequent or severe hypotension and related dizziness or fainting. Increased sensitivity may reflect the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|useInGender= | |useInGender= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
| Line 247: | Line 265: | ||
|overdose= | |overdose= | ||
*Nitroglycerin toxicity is generally mild. The estimated adult oral lethal dose of nitroglycerin is 200 mg to 1,200 mg. Infants may be more susceptible to toxicity from nitroglycerin. Consultation with a poison center should be considered. | |||
*Laboratory determinations of serum levels of nitroglycerin and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of nitroglycerin overdose. | |||
*No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of nitroglycerin and its active metabolites. Similarly, it is not known which if any of these substances can usefully be removed from the body by hemodialysis. | |||
No | *No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary. | ||
*The use of epinephrine or other arterial vasoconstrictors in this setting is not recommended. | |||
*In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of RECTIV overdose in these patients may be subtle and difficult, and invasive monitoring may be required. | |||
=====Methemoglobinemia===== | |||
Methemoglobinemia | *Methemoglobinemia has been rarely reported with organic nitrates. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate arterial PO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air. | ||
*If methemoglobinemia is present, intravenous administration of methylene blue, 1 to 2 mg/kg of body weight, may be required. | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
| Line 276: | Line 295: | ||
|mechAction= | |mechAction= | ||
* | * Nitroglycerin forms free radical nitric oxide (NO), which activates guanylate cyclase, resulting in an increase of guanosine 3',5'-monophosphate (cyclic GMP) in smooth muscle and other tissues. This leads to dephosphorylation of myosin light chains, which regulates the contractile state in smooth muscle and results in vasodilatation. | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure= | ||
* Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is: | *Nitroglycerin is 1,2,3,-propanetriol trinitrate, an organic nitrate whose structural formula is as follows: | ||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
and whose molecular weight is 227.09. RECTIV (nitroglycerin) Ointment 0.4% contains 0.4% nitroglycerin w/w (4 mg nitroglycerin/1 g ointment), propylene glycol, lanolin, sorbitan sesquioleate, paraffin wax, and white petrolatum. RECTIV (nitroglycerin) Ointment 0.4% is available in tubes with a one-inch dosing line on the carton allowing the measurement of approximately 375 mg of nitroglycerin ointment 0.4% (1.5 mg nitroglycerin) for application. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD= | |PD= | ||
*The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Intra-anal application of nitroglycerin reduces sphincter tone and resting intra-anal pressure. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK= | ||
* | *Absorption: In six healthy subjects, the average absolute bioavailability of nitroglycerin applied to the anal canal as a 0.2% w/w ointment was approximately 50% of the 0.75 mg nitroglycerin dose. | ||
*The | *Distribution: The volume of distribution of nitroglycerin following intravenous administration is about 3 L/kg. At plasma concentrations between 50 and 500 ng/mL, the binding of nitroglycerin to plasma proteins is approximately 60%, while that of 1,2- and 1,3-dinitroglycerin is 60% and 30%, respectively. | ||
* | *Metabolism: Nitroglycerin is metabolized by a liver reductase enzyme to glycerol di- and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, the two major metabolites, 1,2- and 1,3- dinitroglycerols are found in plasma. The contribution of metabolites to the relaxation of the internal anal sphincter is unknown. The dinitrates are further metabolized to nonvasoactive mononitrates and ultimately to glycerol and carbon dioxide. | ||
*Elimination: Metabolism is the primary route of drug elimination. Nitroglycerin plasma concentrations decrease rapidly with a mean elimination half-life of two to three minutes. Half-life values range from 1.5 to 7.5 minutes. Clearance (13.6 L/min) greatly exceeds hepatic blood flow.<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |nonClinToxic= | ||
=====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
*Animal carcinogenicity studies with topically applied nitroglycerin have not been performed. | |||
*Rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At the highest dose, the incidence of hepatocellular carcinomas was 52% compared to 0% in untreated controls. Incidence of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice. | |||
*Nitroglycerin was mutagenic in the in vitro bacterial reverse mutation (Ames) assay with Salmonella typhimurium. A similar mutation in this S. typhimurium was also reported with other NO donors. There was no evidence of clastogenic potential in multiple assays including a rodent dominant lethal assay, an in vitro Chinese Hamster Ovary assay that was conducted in the absence of metabolic activation, and several in vivo chromosomal aberration assays conducted in rats and dogs. | |||
* | *In a three-generation reproduction study, rats received dietary nitroglycerin at doses up to approximately 434 mg/kg/day for 6 months prior to mating of the F0 generation with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. | ||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
* | *RECTIV ointment was evaluated in a 3-week double-blind, randomized, multi-center, placebo-controlled study. Patients with a painful chronic anal fissure for at least 6 weeks and moderate or severe pain prior to treatment (≥ 50 mm on the 100 mm visual analog scale, VAS) were randomized to receive 0.4% (1.5 mg) nitroglycerin or placebo ointment applied to the anal canal every 12 hours. Pain as assessed by the change in VAS from baseline to Days 14-18 was lower in patients receiving 0.4% ointment compared to placebo. The mean change from baseline was 44 mm for RECTIV and 37 mm for placebo. The difference in the mean change in pain between RECTIV and placebo was -7.0 mm (95% Confidence Interval: -13.6 to -0.4 mm). | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied= | |howSupplied= | ||
* | *RECTIV (nitroglycerin) Ointment 0.4% is available in 30 g (NDC 42747-235-30) aluminum tubes with polyethylene screw caps. | ||
*Store at 20°-25°C (68°-77°F); excursions permitted between 15°-30°C (59°-86°F). [See USP Controlled Room Temperature]. | |||
*Keep the tube tightly closed. Use within 8 weeks of first opening. | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo= | |fdaPatientInfo= | ||
=====Interaction with PDE5 inhibitors===== | |||
*Advise patient not to use RECTIV with medications for erectile dysfunction such as Viagra (sildenafil), Levitra (vardenafil), and Cialis (tadalafil). These products have been shown to increase the hypotensive effects of RECTIV and other nitrate drugs. | |||
=====Hypotension===== | |||
*Advise patients that treatment with RECTIV may be associated with light-headedness on standing, especially just after rising from a lying or seated position. The effect may be more frequent in patients who have also consumed alcohol, since alcohol use contributes to hypotension. Advise patients to stand up from the supine or sitting position slowly. | |||
=====Headaches===== | |||
*Advise patients that headaches sometimes accompany treatment with RECTIV. For patients who get these headaches, the headaches may indicate the activity of the drug. Tolerance to headaches develops. Advise patients that if they experience headache they should not alter the schedule of their RECTIV treatment to avoid the occurrence of headache. An analgesic, such as acetaminophen, may be used to prevent or relieve the headaches. | |||
=====Dizziness===== | |||
*Advise patients that dizziness has been reported as a side-effect of treatment with RECTIV. | |||
*Advise patients not to drive or operate machinery immediately after applying RECTIV. | |||
=====FDA-Approved Patient Labeling===== | |||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
| Line 351: | Line 381: | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
| Line 364: | Line 393: | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}} | |fileName={{PAGENAME}}05.png|This image is provided by the National Library of Medicine. | ||
}} | }} | ||
Revision as of 14:35, 29 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nitroglycerin (Topical ointment) is a that is FDA approved for the {{{indicationType}}} of . Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- RECTIV™ (nitroglycerin) Ointment 0.4% is indicated for the treatment of moderate to severe pain associated with chronic anal fissure.
Dosage
- Apply 1 inch of ointment (375 mg of ointment equivalent to 1.5 mg of nitroglycerin) intra-anally every 12 hours for up to 3 weeks. A finger covering, such as plastic-wrap, disposable surgical glove or a finger cot, should be placed on the finger to apply the ointment. To obtain a 1.5 mg dose of nitroglycerin, the covered finger is laid alongside the 1 inch dosing line on the carton.
- Refer to carton for accurate dosage guide.
- The tube is gently squeezed until a line of ointment the length of the measuring line is expressed onto the covered finger. The ointment is gently inserted into the anal canal using the covered finger no further than to the first finger joint and the ointment is applied around the side of the anal canal. If this cannot be achieved due to pain, application of the ointment should be made directly to the outside of the anus. Treatment may be continued for up to three weeks.
- RECTIV ointment is not for oral, ophthalmic, or intravaginal use. Hands should be washed after application of the ointment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Topical ointment) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Topical ointment) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Nitroglycerin (Topical ointment) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitroglycerin (Topical ointment) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitroglycerin (Topical ointment) in pediatric patients.
Contraindications
PDE5 inhibitor use
- Administration of RECTIV is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), such as sildenafil, vardenafil, and tadalafil, as these are shown to potentiate the hypotensive effects of organic nitrates [see 7.1 DRUG INTERACTIONS].
=====Severe anemia
=
- RECTIV is contraindicated in patients with severe anemia.
=====Increased intracranial pressure
=
- RECTIV is contraindicated in patients with increased intracranial pressure.
=====Hypersensitivity
=
- RECTIV is contraindicated in patients who have shown hypersensitivity to it or to other nitrates or nitrites. Skin reactions consistent with hypersensitivity have been observed with organic nitrates.
Warnings
Cardiovascular disorders
- Venous and arterial dilatation as a consequence of nitroglycerin treatment including RECTIV, can decrease venous blood returning to the heart and reduce arterial vascular resistance and systolic pressure. Exercise caution when treating patients with any of the following conditions: blood volume depletion, existing hypotension, cardiomyopathies, congestive heart failure, acute myocardial infarction, or poor cardiac function for other reasons. If patients with any of these conditions are treated with RECTIV, monitor cardiovascular status and clinical condition. The adverse reactions of RECTIV are likely to be more pronounced in the elderly.
Headache
- RECTIV produces dose-related headaches, which may be severe. Tolerance to headaches occurs.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The most common adverse reaction of RECTIV (nitroglycerin) Ointment 0.4% applied to the anal canal is headache.
- Headache may be recurrent following each dose. Headaches are typically of short duration and can be treated with an analgesic, e.g. acetaminophen, and are reversible upon discontinuation of treatment.
- In Study REC-C-001, a double-blind, placebo-controlled trial in patients with a painful chronic anal fissure, the most frequent (≥ 2%) adverse reactions reported were as follows (Table 1):
Hypotension
- Transient episodes of light-headedness, occasionally related to blood pressure changes, also may occur. Hypotension (including orthostatic hypotension) occurs infrequently, but in some patients may be severe enough to warrant discontinuation of therapy.
Allergic Reactions
- Flushing, allergic reactions and application site reactions (including drug rash and exfoliative dermatitis) have been reported rarely.
Methemoglobinemia
- In rare cases, therapeutic doses of organic nitrates have caused methemoglobinemia
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nitroglycerin (Topical ointment) in the drug label.
Drug Interactions
PDE5 inhibitors
- Phosphodiesterase type 5 (PDE5) inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates.
- The time course of the interaction appears to be related to the half-life of the PDE5 inhibitor, however, the dose dependence of this interaction has not been studied. Use of RECTIV within a few days of PDE5 inhibitors is contraindicated.
Antihypertensives
- Patients receiving antihypertensive drugs, beta-adrenergic blockers, and other nitrates should be observed for possible additive hypotensive effects when using RECTIV. Marked orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used concomitantly.
- Beta-blockers blunt the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effects. If beta-blockers are used with RECTIV in patients with angina pectoris, additional hypotensive effects may occur.
Aspirin
- Coadministration of aspirin (at doses between 500 mg and 1000 mg) and nitroglycerin has been reported to result in increased nitroglycerin maximum concentrations by as much as 67% and AUC by 73% when administered as a single dose. The pharmacological effects of RECTIV may be enhanced by concomitant administration of aspirin.
Tissue-type Plasminogen Activator (t-PA)
- Intravenous administration of nitroglycerin decreases the thrombolytic effect of tissue-type plasminogen activator (t-PA). Plasma levels of t-PA are reduced when coadministered with nitroglycerin. Therefore, caution should be observed in patients receiving RECTIV during t-PA therapy.
Heparin
- Although an interaction has been reported between intravenous heparin and intravenous nitroglycerin (resulting in a decrease in the anticoagulant effect of heparin), the data are not consistent. If patients are to receive intravenous heparin and RECTIV concurrently, the anticoagulation status of the patient must be checked.
Ergotamine
- Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and consequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore the possibility of ergotism in patients receiving RECTIV should be considered.
Alcohol
- The vasodilating effects of nitroglycerin have been shown to be additive to the effects observed with alcohol.
Use in Specific Populations
Pregnancy
- Pregnancy Category CAnimal reproduction and teratogenicity studies have not been conducted with RECTIV. Nitroglycerin was not teratogenic when administered by topical or dietary route. There are no adequate and well-controlled studies in pregnant women. RECTIV should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Teratology studies in rats and rabbits were conducted with topically applied nitroglycerin ointment at doses up to 80 mg/kg/day and 240 mg/kg/day, respectively. No toxic effects on dams or fetuses were seen at any dose tested.
- A teratogenicity study was conducted in rats with nitroglycerin administered in the diet at levels up to 1% content (approximately 430 mg/kg/day) on days 6 to 15 of gestation. In offspring of the high-dose group, an increased but not statistically significant incidence of diaphragmatic hernias was noted together with decreased hyoid bone ossification. The latter finding probably reflects delayed development, thus indicating no clear evidence of a potential teratogenic effect of nitroglycerin.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitroglycerin (Topical ointment) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nitroglycerin (Topical ointment) during labor and delivery.
Nursing Mothers
- It is not known whether nitroglycerin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when RECTIV is administered to a nursing woman.
Pediatric Use
- The safety and effectiveness of RECTIV in pediatric patients under 18 years of age have not been established.
Geriatic Use
- Clinical studies of RECTIV did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Clinical data from the published literature indicate that the elderly demonstrate increased sensitivity to nitrates, which may be therapeutic but also manifest by more frequent or severe hypotension and related dizziness or fainting. Increased sensitivity may reflect the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nitroglycerin (Topical ointment) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nitroglycerin (Topical ointment) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Nitroglycerin (Topical ointment) in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Nitroglycerin (Topical ointment) in the drug label.
Overdosage
- Nitroglycerin toxicity is generally mild. The estimated adult oral lethal dose of nitroglycerin is 200 mg to 1,200 mg. Infants may be more susceptible to toxicity from nitroglycerin. Consultation with a poison center should be considered.
- Laboratory determinations of serum levels of nitroglycerin and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of nitroglycerin overdose.
- No data are available to suggest physiological maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of nitroglycerin and its active metabolites. Similarly, it is not known which if any of these substances can usefully be removed from the body by hemodialysis.
- No specific antagonist to the vasodilator effects of nitroglycerin is known, and no intervention has been subject to controlled study as a therapy of nitroglycerin overdose. Because the hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. Passive elevation of the patient's legs may be sufficient, but intravenous infusion of normal saline or similar fluid may also be necessary.
- The use of epinephrine or other arterial vasoconstrictors in this setting is not recommended.
- In patients with renal disease or congestive heart failure, therapy resulting in central volume expansion is not without hazard. Treatment of RECTIV overdose in these patients may be subtle and difficult, and invasive monitoring may be required.
Methemoglobinemia
- Methemoglobinemia has been rarely reported with organic nitrates. The diagnosis should be suspected in patients who exhibit signs of impaired oxygen delivery despite adequate arterial PO2. Classically, methemoglobinemic blood is described as chocolate brown, without color change on exposure to air.
- If methemoglobinemia is present, intravenous administration of methylene blue, 1 to 2 mg/kg of body weight, may be required.
Pharmacology
There is limited information regarding Nitroglycerin (Topical ointment) Pharmacology in the drug label.
Mechanism of Action
- Nitroglycerin forms free radical nitric oxide (NO), which activates guanylate cyclase, resulting in an increase of guanosine 3',5'-monophosphate (cyclic GMP) in smooth muscle and other tissues. This leads to dephosphorylation of myosin light chains, which regulates the contractile state in smooth muscle and results in vasodilatation.
Structure
- Nitroglycerin is 1,2,3,-propanetriol trinitrate, an organic nitrate whose structural formula is as follows:
and whose molecular weight is 227.09. RECTIV (nitroglycerin) Ointment 0.4% contains 0.4% nitroglycerin w/w (4 mg nitroglycerin/1 g ointment), propylene glycol, lanolin, sorbitan sesquioleate, paraffin wax, and white petrolatum. RECTIV (nitroglycerin) Ointment 0.4% is available in tubes with a one-inch dosing line on the carton allowing the measurement of approximately 375 mg of nitroglycerin ointment 0.4% (1.5 mg nitroglycerin) for application.
Pharmacodynamics
- The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Intra-anal application of nitroglycerin reduces sphincter tone and resting intra-anal pressure.
Pharmacokinetics
- Absorption: In six healthy subjects, the average absolute bioavailability of nitroglycerin applied to the anal canal as a 0.2% w/w ointment was approximately 50% of the 0.75 mg nitroglycerin dose.
- Distribution: The volume of distribution of nitroglycerin following intravenous administration is about 3 L/kg. At plasma concentrations between 50 and 500 ng/mL, the binding of nitroglycerin to plasma proteins is approximately 60%, while that of 1,2- and 1,3-dinitroglycerin is 60% and 30%, respectively.
- Metabolism: Nitroglycerin is metabolized by a liver reductase enzyme to glycerol di- and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, the two major metabolites, 1,2- and 1,3- dinitroglycerols are found in plasma. The contribution of metabolites to the relaxation of the internal anal sphincter is unknown. The dinitrates are further metabolized to nonvasoactive mononitrates and ultimately to glycerol and carbon dioxide.
- Elimination: Metabolism is the primary route of drug elimination. Nitroglycerin plasma concentrations decrease rapidly with a mean elimination half-life of two to three minutes. Half-life values range from 1.5 to 7.5 minutes. Clearance (13.6 L/min) greatly exceeds hepatic blood flow.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal carcinogenicity studies with topically applied nitroglycerin have not been performed.
- Rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At the highest dose, the incidence of hepatocellular carcinomas was 52% compared to 0% in untreated controls. Incidence of testicular tumors were 52% vs. 8% in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
- Nitroglycerin was mutagenic in the in vitro bacterial reverse mutation (Ames) assay with Salmonella typhimurium. A similar mutation in this S. typhimurium was also reported with other NO donors. There was no evidence of clastogenic potential in multiple assays including a rodent dominant lethal assay, an in vitro Chinese Hamster Ovary assay that was conducted in the absence of metabolic activation, and several in vivo chromosomal aberration assays conducted in rats and dogs.
- In a three-generation reproduction study, rats received dietary nitroglycerin at doses up to approximately 434 mg/kg/day for 6 months prior to mating of the F0 generation with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males.
Clinical Studies
- RECTIV ointment was evaluated in a 3-week double-blind, randomized, multi-center, placebo-controlled study. Patients with a painful chronic anal fissure for at least 6 weeks and moderate or severe pain prior to treatment (≥ 50 mm on the 100 mm visual analog scale, VAS) were randomized to receive 0.4% (1.5 mg) nitroglycerin or placebo ointment applied to the anal canal every 12 hours. Pain as assessed by the change in VAS from baseline to Days 14-18 was lower in patients receiving 0.4% ointment compared to placebo. The mean change from baseline was 44 mm for RECTIV and 37 mm for placebo. The difference in the mean change in pain between RECTIV and placebo was -7.0 mm (95% Confidence Interval: -13.6 to -0.4 mm).
How Supplied
- RECTIV (nitroglycerin) Ointment 0.4% is available in 30 g (NDC 42747-235-30) aluminum tubes with polyethylene screw caps.
- Store at 20°-25°C (68°-77°F); excursions permitted between 15°-30°C (59°-86°F). [See USP Controlled Room Temperature].
- Keep the tube tightly closed. Use within 8 weeks of first opening.
Storage
There is limited information regarding Nitroglycerin (Topical ointment) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Nitroglycerin (Topical ointment) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nitroglycerin (Topical ointment) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Interaction with PDE5 inhibitors
- Advise patient not to use RECTIV with medications for erectile dysfunction such as Viagra (sildenafil), Levitra (vardenafil), and Cialis (tadalafil). These products have been shown to increase the hypotensive effects of RECTIV and other nitrate drugs.
Hypotension
- Advise patients that treatment with RECTIV may be associated with light-headedness on standing, especially just after rising from a lying or seated position. The effect may be more frequent in patients who have also consumed alcohol, since alcohol use contributes to hypotension. Advise patients to stand up from the supine or sitting position slowly.
Headaches
- Advise patients that headaches sometimes accompany treatment with RECTIV. For patients who get these headaches, the headaches may indicate the activity of the drug. Tolerance to headaches develops. Advise patients that if they experience headache they should not alter the schedule of their RECTIV treatment to avoid the occurrence of headache. An analgesic, such as acetaminophen, may be used to prevent or relieve the headaches.
Dizziness
- Advise patients that dizziness has been reported as a side-effect of treatment with RECTIV.
- Advise patients not to drive or operate machinery immediately after applying RECTIV.
FDA-Approved Patient Labeling
Precautions with Alcohol
- Alcohol-Nitroglycerin (Topical ointment) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
There is limited information regarding Nitroglycerin (Topical ointment) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Nitroglycerin (Topical ointment) |Label Name=Nitroglycerin (Topical ointment)04.png
}}
{{#subobject:
|Label Page=Nitroglycerin (Topical ointment) |Label Name=Nitroglycerin (Topical ointment)05.png
}}