Loracarbef: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | {{Drugbox | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 49: | Line 48: | ||
| StdInChIKey = GPYKKBAAPVOCIW-HSASPSRMSA-N | | StdInChIKey = GPYKKBAAPVOCIW-HSASPSRMSA-N | ||
}} | }} | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} | ||
Revision as of 17:39, 13 April 2015
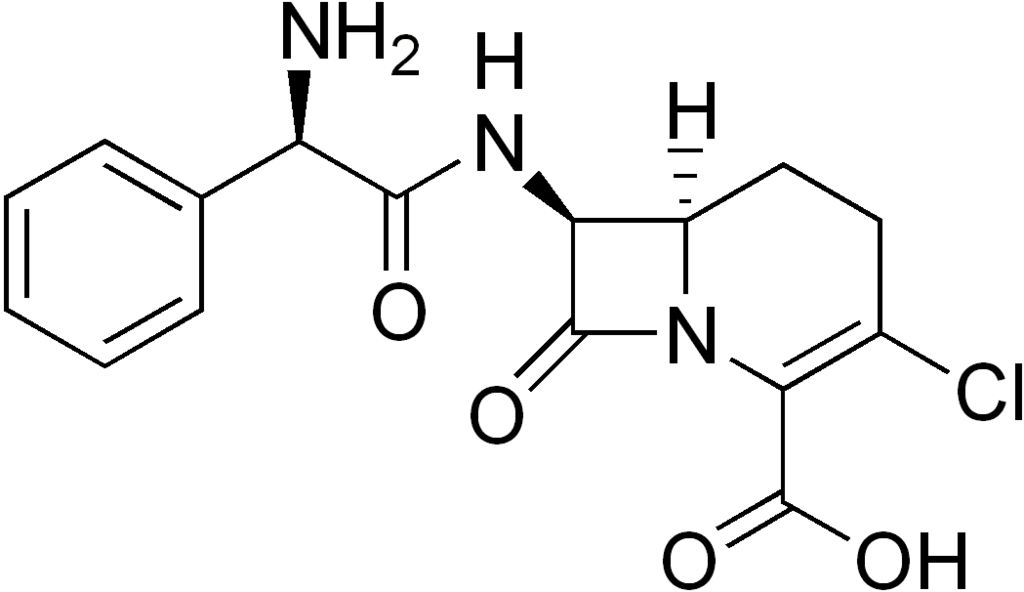 | |
| Clinical data | |
|---|---|
| Trade names | Lorabid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 25% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H16ClN3O4 |
| Molar mass | 349.769 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Loracarbef |
|
Articles |
|---|
|
Most recent articles on Loracarbef |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Loracarbef at Clinical Trials.gov Clinical Trials on Loracarbef at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Loracarbef
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Loracarbef Discussion groups on Loracarbef Patient Handouts on Loracarbef Directions to Hospitals Treating Loracarbef Risk calculators and risk factors for Loracarbef
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Loracarbef |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Loracarbef is a synthetic carbacephem antibiotic.[1] Loracarbef has a spectrum of activity similar to that of the second generation cephalosporins. It is an analogue of cefaclor from which the sulfur atom is replaced with a methylene group. Therefore loracarbef has a greater stability in solution and may be stored at room temperature.
Diarrhea is the most common adverse effect. Side effects are more frequently seen with children under the age of twelve. It received FDA approval in 1991. Its use was discontinued in 2006.
Category
Carbacephem
Brand Names
LORABID® (DISCONTINUED)
Mechanism of Action
Loracarbef is an oral, synthetic beta-lactam antibiotic of the carbacephem class. Loracarbef, like all beta-lactams and cephalosporins, inhibits penicillin binding proteins, enzymes that create the cross-linkage of the peptidoglycan polymer. This binding leads to interference with the formation and remodeling of the cell wall structure.
References
- ↑ Biedenbach DJ, Jones RN (1994). "Predictive accuracy of disk diffusion test for Proteus vulgaris and Providencia species against five newer orally administered cephalosporins, cefdinir, cefetamet, cefprozil, cefuroxime, and loracarbef". J. Clin. Microbiol. 32 (2): 559–62. PMC 263078. PMID 8150976. Unknown parameter
|month=ignored (help)
- Pages with script errors
- Pages with citations using unsupported parameters
- Template:drugs.com link with non-standard subpage
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Antibiotics
- Drug