Tolperisone: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | {{Drugbox | ||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 451425253 | |||
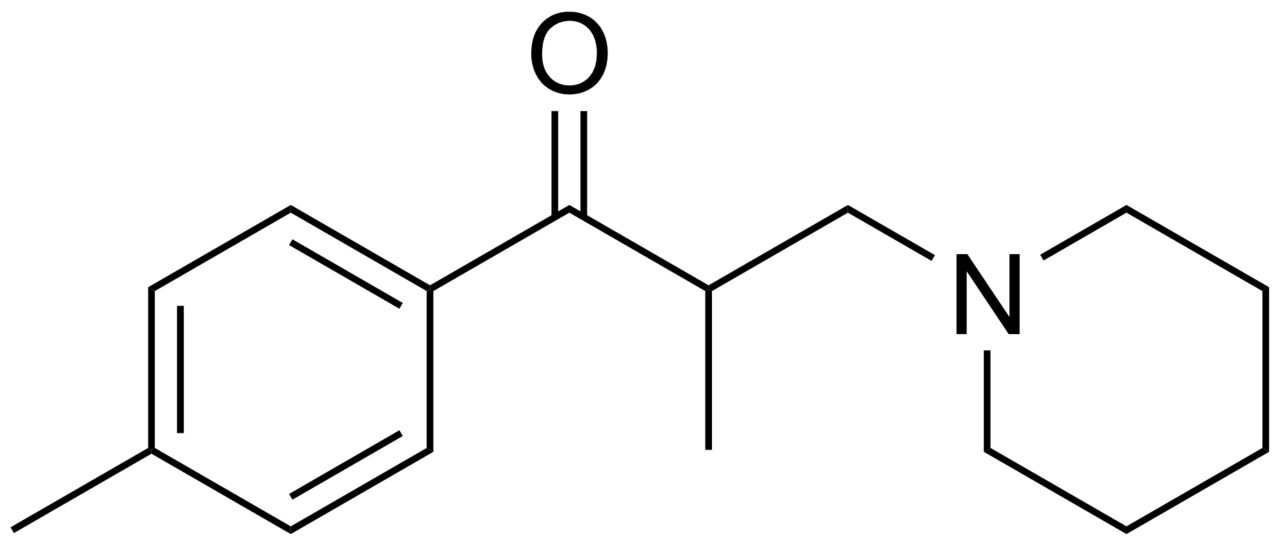
| IUPAC_name = 2-methyl-1-(4-methylphenyl)-3-(1-piperidyl)propan-1-one | | IUPAC_name = 2-methyl-1-(4-methylphenyl)-3-(1-piperidyl)propan-1-one | ||
| image=Tolperisone. | | image = Tolperisone-2d-skeletal.png | ||
| width = | | width = 150 | ||
| CAS_number=728-88-1 | |||
| ATC_prefix= | <!--Clinical data--> | ||
| ATC_suffix= | | tradename = Mydocalm and others | ||
| ATC_supplemental= | | Drugs.com = {{drugs.com|international|tolperisone}} | ||
| PubChem=5511 | | pregnancy_category= | ||
| | | legal_status = Rx-only | ||
| C = 16 | H = 23 | N = 1 | O = 1 | | routes_of_administration = Oral, [[parenteral]] | ||
<!-- Pharmacokinetic data --> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = Liver, kidney | |||
| elimination_half-life = 1st phase: 2 hrs<br />2nd phase: 12 hrs | |||
| excretion = [[Kidney|Renal]] | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 728-88-1 | |||
| ATC_prefix = M02 | |||
| ATC_suffix = AX06 | |||
| ATC_supplemental = {{ATC|M03|BX04}} | |||
| PubChem = 5511 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1076211 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = F5EOM0LD8E | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08617 | |||
<!--Chemical data--> | |||
| C=16 | H=23 | N=1 | O=1 | |||
| molecular_weight = 245.36 g/mol | | molecular_weight = 245.36 g/mol | ||
| | | smiles = C1CCCN(C1)CC(C(C2=CC=C(C=C2)C)=O)C | ||
| | | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | ||
| | | StdInChI = 1S/C16H23NO/c1-13-6-8-15(9-7-13)16(18)14(2)12-17-10-4-3-5-11-17/h6-9,14H,3-5,10-12H2,1-2H3 | ||
| | | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | ||
| | | StdInChIKey = FSKFPVLPFLJRQB-UHFFFAOYSA-N | ||
| | |||
| | |||
}} | }} | ||
'''Tolperisone''', a [[piperidine]] derivative, is a centrally acting [[muscle relaxant]]. Trade names include '''Biocalm''', '''Muscodol''', '''Mydeton''', '''Mydocalm''', '''Myolax''', '''Myoxan''' and '''Viveo'''. | |||
{{ | |||
{{ | == Clinical use == | ||
In Tolperisone is indicated for use in the treatment of pathologically increased tone of the cross-striated muscle caused by neurological diseases (damage of the [[pyramidal tract]], [[multiple sclerosis]], [[myelopathy]], [[encephalomyelitis]]) and of spastic paralysis and other encephalopathies manifested with muscular dystonia.<ref name="Austria-Codex">{{cite book|title=Austria-Codex|editor=Jasek, W|publisher=Österreichischer Apothekerverlag|location=Vienna|year=2007|edition=62nd|pages=5510–1|isbn=978-3-85200-181-4|language=German}}</ref><ref name="Midocalm-ro">{{ cite web | url = http://www.info-medic.ro/midocalm/ | title = Midocalm | publisher = InfoMedic| location = Romania}}</ref> | |||
Other possible uses include:{{fact|date=May 2012}} | |||
*[[Spondylosis]] | |||
*Spondylarthrosis | |||
*Cervical and lumbar syndromes | |||
*[[Arthrosis]] of the large joints | |||
*[[atherosclerosis|Obliterating atherosclerosis]] of the extremity vessels | |||
*[[Diabetic angiopathy]] | |||
*[[Thromboangiitis obliterans]] | |||
*[[Raynaud's syndrome]] | |||
== Contraindications and cautions == | |||
Manufacturers report that tolperisone should not be used in patients with [[myasthenia gravis]]. Only limited data are available regarding the safety in children, youths, during pregnancy and breastfeeding. It is not known whether tolperisone is excreted into mother's milk.<ref name="Austria-Codex" /><ref name="Midocalm-ro" /> | |||
== Side effects == | |||
Adverse effects occur in fewer than 1% of patients and include muscle weakness, headache, arterial [[hypotension]], nausea, vomiting, [[dyspepsia]], and dry mouth. All effects are reversible.<ref name="Austria-Codex" /><ref name="Midocalm-ro" /> | |||
Allergic reactions occur in fewer than 0.1% of patient and include skin rash, [[hives]], [[Quincke's edema]], and in some cases [[anaphylactic shock]].<ref name="Austria-Codex" /><ref>{{ cite journal | author = Ribi C, Vermeulen C, Hauser C | title = Anaphylactic reactions to tolperisone (Mydocalm) | journal = Swiss Medical Weekly | volume = 133 | issue = 25–26 | pages = 369–371 | year = 2003 | pmid = 12947534 }}</ref><ref name="pmid14587432">{{cite journal |author=Kwaśniewski A, Korbuszewska-Gontarz B, Mika S |title=[Mydocalm causing anaphylaxis] |language=Polish |journal=Pneumonol Alergol Pol |volume=71 |issue=5-6 |pages=250–2 |year=2003 |pmid=14587432 |doi= |url= |accessdate=2014-12-17}}</ref><ref name="pmid21905508">{{cite journal |author=Glück J, Rymarczyk B, Rogala B |title=An immediate hypersensitivity reaction caused by tolperisone hydrochloride |journal=J Investig Allergol Clin Immunol |volume=21 |issue=5 |pages=411–2 |year=2011 |pmid=21905508 |doi= |url= |accessdate=2014-12-17}}</ref> | |||
== Overdose == | |||
Excitability has been noted after ingestion of high doses by children.<ref name="Austria-Codex" /> | |||
In suicide studies of three isolated cases, it is believed that ingestion of tolperisone was the cause of death.<ref>{{ cite journal | author = Sporkert, F.; Brunel, C.; Augsburger, M. P.; Mangin, P. | title = Fatal tolperisone poisoning: Autopsy and toxicology findings in three suicide cases | journal = Forensic Science International | year = 2012 | volume = 215 | issue = 1 | pages = 101–104 | doi = 10.1016/j.forsciint.2011.05.025 | pmid = 21683537 }}</ref> | |||
== Interactions == | |||
Tolperisone does not have a significant potential for interactions with other pharmaceutical drugs. It cannot be excluded that combination with other centrally acting muscle relaxants, [[benzodiazepine]]s or [[non-steroidal anti-inflammatory drug]]s (NSAIDs) may make a dose reduction necessary in some patients.<ref name="Austria-Codex" /><ref name="Midocalm-ro" /> | |||
== Mechanism of action == | |||
Tolperisone is a centrally acting muscle relaxant that acts at the [[reticular formation]] in the brain stem<ref name="Austria-Codex" /> by blocking [[Voltage-gated sodium channel|voltage-gated sodium]] and [[Voltage-gated calcium channel|calcium channels]].<ref>{{ cite journal | author = Kocsis P, Farkas S, Fodor L, Bielik N, Thán M, Kolok S, Gere A, Csejtei M, Tarnawa I | title = Tolperisone-type drugs inhibit spinal reflexes via blockade of voltage-gated sodium and calcium channels | journal = Journal of Pharmacology and Experimental Therapeutics | volume = 315 | issue = 3 | pages = 1237–1246 | year = 2005 | pmid = 16126840 | doi = 10.1124/jpet.105.089805 }}</ref><ref>{{ cite journal | author = Hofer D, Lohberger B, Steinecker B, Schmidt K, Quasthoff S, Schreibmayer W | title = A comparative study of the action of tolperisone on seven different voltage dependent sodium channel isoforms | journal = European Journal of Pharmacology | volume = 538 | issue = 1–3 | pages = 5–14 | year = 2006 | pmid = 16650844 | doi = 10.1016/j.ejphar.2006.03.034 }}</ref> | |||
== Pharmacokinetics == | |||
Tolperisone is absorbed nearly completely from the gut and reaches its [[peak (pharmacology)|peak]] blood plasma concentration after 1.5 hours. It is extensively metabolised in the liver and kidneys. The substance is excreted via the kidneys in two phases; the first with a half-life of two hours, and the second with a half-life of 12 hours.<ref name="Austria-Codex" /> | |||
== See also == | |||
* Chemically and mechanistically related drugs: [[eperisone]], [[inaperisone]], [[lanperisone]], [[silperisone]] | |||
== | == References == | ||
{{reflist|2}} | |||
==External links== | == External links == | ||
* {{cite | * {{ cite web | url = http://lib.bioinfo.pl/meid:189350 | title = Tolperisone: Administration & Dosage - Papers | publisher = BioInfo | location = Poland }}{{dead link|date=December 2013}} | ||
{{Muscle relaxants}} | {{Muscle relaxants}} | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Piperidines]] | |||
[[Category:Muscle relaxants]] | |||
[[Category:Aromatic ketones]] | |||
Revision as of 12:50, 10 April 2015
 | |
| Clinical data | |
|---|---|
| Trade names | Mydocalm and others |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver, kidney |
| Elimination half-life | 1st phase: 2 hrs 2nd phase: 12 hrs |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C16H23NO |
| Molar mass | 245.36 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Tolperisone, a piperidine derivative, is a centrally acting muscle relaxant. Trade names include Biocalm, Muscodol, Mydeton, Mydocalm, Myolax, Myoxan and Viveo.
Clinical use
In Tolperisone is indicated for use in the treatment of pathologically increased tone of the cross-striated muscle caused by neurological diseases (damage of the pyramidal tract, multiple sclerosis, myelopathy, encephalomyelitis) and of spastic paralysis and other encephalopathies manifested with muscular dystonia.[1][2]
Other possible uses include:[citation needed]
- Spondylosis
- Spondylarthrosis
- Cervical and lumbar syndromes
- Arthrosis of the large joints
- Obliterating atherosclerosis of the extremity vessels
- Diabetic angiopathy
- Thromboangiitis obliterans
- Raynaud's syndrome
Contraindications and cautions
Manufacturers report that tolperisone should not be used in patients with myasthenia gravis. Only limited data are available regarding the safety in children, youths, during pregnancy and breastfeeding. It is not known whether tolperisone is excreted into mother's milk.[1][2]
Side effects
Adverse effects occur in fewer than 1% of patients and include muscle weakness, headache, arterial hypotension, nausea, vomiting, dyspepsia, and dry mouth. All effects are reversible.[1][2] Allergic reactions occur in fewer than 0.1% of patient and include skin rash, hives, Quincke's edema, and in some cases anaphylactic shock.[1][3][4][5]
Overdose
Excitability has been noted after ingestion of high doses by children.[1] In suicide studies of three isolated cases, it is believed that ingestion of tolperisone was the cause of death.[6]
Interactions
Tolperisone does not have a significant potential for interactions with other pharmaceutical drugs. It cannot be excluded that combination with other centrally acting muscle relaxants, benzodiazepines or non-steroidal anti-inflammatory drugs (NSAIDs) may make a dose reduction necessary in some patients.[1][2]
Mechanism of action
Tolperisone is a centrally acting muscle relaxant that acts at the reticular formation in the brain stem[1] by blocking voltage-gated sodium and calcium channels.[7][8]
Pharmacokinetics
Tolperisone is absorbed nearly completely from the gut and reaches its peak blood plasma concentration after 1.5 hours. It is extensively metabolised in the liver and kidneys. The substance is excreted via the kidneys in two phases; the first with a half-life of two hours, and the second with a half-life of 12 hours.[1]
See also
- Chemically and mechanistically related drugs: eperisone, inaperisone, lanperisone, silperisone
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 5510–1. ISBN 978-3-85200-181-4.
- ↑ 2.0 2.1 2.2 2.3 "Midocalm". Romania: InfoMedic.
- ↑ Ribi C, Vermeulen C, Hauser C (2003). "Anaphylactic reactions to tolperisone (Mydocalm)". Swiss Medical Weekly. 133 (25–26): 369–371. PMID 12947534.
- ↑ Kwaśniewski A, Korbuszewska-Gontarz B, Mika S (2003). "[Mydocalm causing anaphylaxis]". Pneumonol Alergol Pol (in Polish). 71 (5–6): 250–2. PMID 14587432.
|access-date=requires|url=(help) - ↑ Glück J, Rymarczyk B, Rogala B (2011). "An immediate hypersensitivity reaction caused by tolperisone hydrochloride". J Investig Allergol Clin Immunol. 21 (5): 411–2. PMID 21905508.
|access-date=requires|url=(help) - ↑ Sporkert, F.; Brunel, C.; Augsburger, M. P.; Mangin, P. (2012). "Fatal tolperisone poisoning: Autopsy and toxicology findings in three suicide cases". Forensic Science International. 215 (1): 101–104. doi:10.1016/j.forsciint.2011.05.025. PMID 21683537.
- ↑ Kocsis P, Farkas S, Fodor L, Bielik N, Thán M, Kolok S, Gere A, Csejtei M, Tarnawa I (2005). "Tolperisone-type drugs inhibit spinal reflexes via blockade of voltage-gated sodium and calcium channels". Journal of Pharmacology and Experimental Therapeutics. 315 (3): 1237–1246. doi:10.1124/jpet.105.089805. PMID 16126840.
- ↑ Hofer D, Lohberger B, Steinecker B, Schmidt K, Quasthoff S, Schreibmayer W (2006). "A comparative study of the action of tolperisone on seven different voltage dependent sodium channel isoforms". European Journal of Pharmacology. 538 (1–3): 5–14. doi:10.1016/j.ejphar.2006.03.034. PMID 16650844.
External links
- "Tolperisone: Administration & Dosage - Papers". Poland: BioInfo.[dead link]
- Pages with script errors
- CS1 maint: Unrecognized language
- CS1 maint: Multiple names: authors list
- Pages using citations with accessdate and no URL
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All articles with unsourced statements
- Articles with unsourced statements from May 2012
- Articles with invalid date parameter in template
- All articles with dead external links
- Articles with dead external links from December 2013
- Drug
- Piperidines
- Muscle relaxants
- Aromatic ketones