Carbamide: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
Rabin Bista (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{RB}} | |||
|OTC=Yes | |||
|genericName=Carbamide | |||
|aOrAn=a | |aOrAn=a | ||
|drugClass=OTC emollient | |||
|indicationType=treatment | |indicationType=treatment | ||
| | |indication=excessive ear wax | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions=allergic reactions | ||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult===== | |fdaLIADAdult=====Indications==== | ||
* For occasional use as an aid to soften, loosen, and remove excessive ear wax. | |||
====Dosage==== | |||
* FOR USE IN THE EAR ONLY. Adults and children over 12 years of age: tilt head sideways and place 5 to 10 drops into ear. Tip of applicator should not enter ear canal. Keep drops in ear for several minutes by keeping head tilted or placing cotton in ear. Use twice daily for up to four days if needed, or as directed by a doctor. Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe. Children under 12 years of age: consult a doctor. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
==== | |||
* | |||
: | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed===== | |fdaLIADPed=====Indications==== | ||
* For occasional use as an aid to soften, loosen, and remove excessive ear wax. | |||
* | |||
====Dosage==== | |||
* FOR USE IN THE EAR ONLY. Adults and children over 12 years of age: tilt head sideways and place 5 to 10 drops into ear. Tip of applicator should not enter ear canal. Keep drops in ear for several minutes by keeping head tilted or placing cotton in ear. Use twice daily for up to four days if needed, or as directed by a doctor. Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe. Children under 12 years of age: consult a doctor. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
==== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications= | |contraindications= | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings=Do not use: if you have ear drainage or discharge, ear pain, irritation or rash in the ear, or are dizzy; consult a doctor. If you have an injury or perforation (hole) of the eardrum or after ear surgery, unless directed by a doctor. For more than four consecutive days. | ||
When using this product: avoid contact with the eyes. | |||
Stop use and ask a doctor if: excessive ear wax remains after use of this product for four consecutive days. | |||
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. If accidental contact with eyes occurs, flush eyes with water and consult a doctor. | |||
|clinicalTrials=* allergic reactions | |||
= | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
| Line 244: | Line 107: | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions= | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
| Line 264: | Line 126: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* topical ear drops | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 275: | Line 135: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=There is limited information regarding <i>Overdose</i> of {{PAGENAME}} in the drug label. | ||
There is limited information regarding <i> | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
| Line 296: | Line 144: | ||
<!--Structure--> | <!--Structure--> | ||
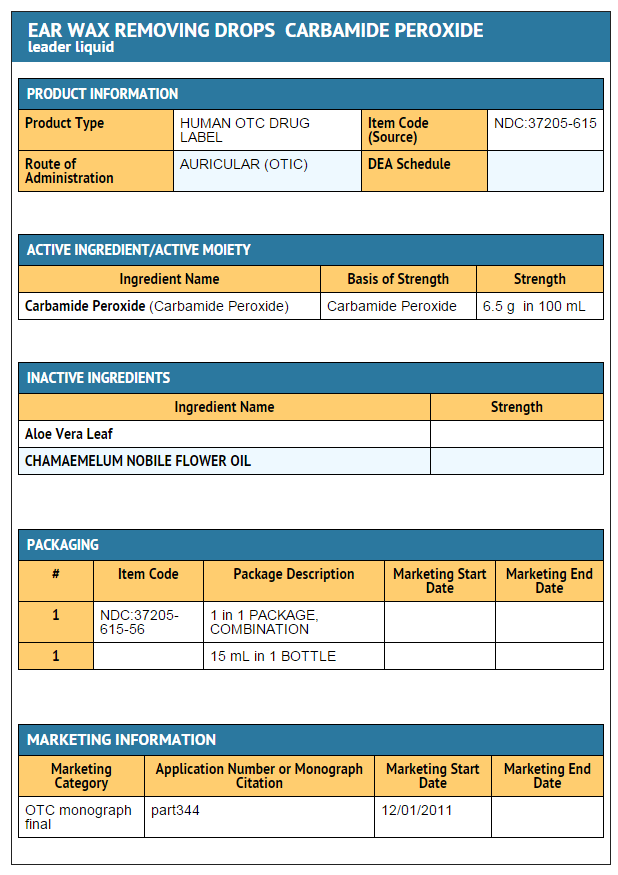
|structure=* | |structure=* Active Ingredient | ||
Carbamide Peroxide 6.5% | |||
Inactive ingredients | |||
Glycerin, Oxyquinoline, Aloe Barbadensis Leaf Extract, Anthemis Nobilis Flower Oil | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 314: | Line 168: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* | ||
|packLabel=<!--Patient Counseling Information--> | |packLabel=PRINCIPAL DISPLAY PANEL | ||
Ear Wax Cleansing Kit | |||
Step 1 Ear Wax Removing Drops | |||
Step 2 Ear Cleansing Spray | |||
: [[File:Carbamide PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File:Carbamide Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
| Line 321: | Line 188: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* EAR WAX REMOVING DROPS CARBAMIDE PEROXIDE®<ref>{{Cite web | title = CARBAMIDE PEROXIDE | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1a1523f0-9d65-44de-afab-a0df6faebb8a}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
Revision as of 13:28, 9 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Carbamide is a OTC emollient that is FDA approved for the treatment of excessive ear wax. Common adverse reactions include allergic reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- For occasional use as an aid to soften, loosen, and remove excessive ear wax.
Dosage
- FOR USE IN THE EAR ONLY. Adults and children over 12 years of age: tilt head sideways and place 5 to 10 drops into ear. Tip of applicator should not enter ear canal. Keep drops in ear for several minutes by keeping head tilted or placing cotton in ear. Use twice daily for up to four days if needed, or as directed by a doctor. Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe. Children under 12 years of age: consult a doctor.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carbamide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carbamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- For occasional use as an aid to soften, loosen, and remove excessive ear wax.
Dosage
- FOR USE IN THE EAR ONLY. Adults and children over 12 years of age: tilt head sideways and place 5 to 10 drops into ear. Tip of applicator should not enter ear canal. Keep drops in ear for several minutes by keeping head tilted or placing cotton in ear. Use twice daily for up to four days if needed, or as directed by a doctor. Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe. Children under 12 years of age: consult a doctor.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carbamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carbamide in pediatric patients.
Contraindications
There is limited information regarding Carbamide Contraindications in the drug label.
Warnings
Do not use: if you have ear drainage or discharge, ear pain, irritation or rash in the ear, or are dizzy; consult a doctor. If you have an injury or perforation (hole) of the eardrum or after ear surgery, unless directed by a doctor. For more than four consecutive days. When using this product: avoid contact with the eyes. Stop use and ask a doctor if: excessive ear wax remains after use of this product for four consecutive days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. If accidental contact with eyes occurs, flush eyes with water and consult a doctor.
Adverse Reactions
Clinical Trials Experience
- allergic reactions
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Carbamide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
There is limited information regarding Carbamide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Carbamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Carbamide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Carbamide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Carbamide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Carbamide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Carbamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carbamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Carbamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Carbamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Carbamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Carbamide in patients who are immunocompromised.
Administration and Monitoring
Administration
- topical ear drops
Monitoring
There is limited information regarding Monitoring of Carbamide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Carbamide in the drug label.
Overdosage
There is limited information regarding Overdose of Carbamide in the drug label.
Pharmacology
There is limited information regarding Carbamide Pharmacology in the drug label.
Mechanism of Action
Structure
- Active Ingredient
Carbamide Peroxide 6.5%
Inactive ingredients
Glycerin, Oxyquinoline, Aloe Barbadensis Leaf Extract, Anthemis Nobilis Flower Oil
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Carbamide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Carbamide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Carbamide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Carbamide in the drug label.
How Supplied
Storage
There is limited information regarding Carbamide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Carbamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL Ear Wax Cleansing Kit
Step 1 Ear Wax Removing Drops
Step 2 Ear Cleansing Spray
Ingredients and Appearance
{{#ask: Label Page::Carbamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Carbamide in the drug label.
Precautions with Alcohol
- Alcohol-Carbamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- EAR WAX REMOVING DROPS CARBAMIDE PEROXIDE®[1]
Look-Alike Drug Names
There is limited information regarding Carbamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Carbamide
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Carbamide |Label Name=Carbamide11.png
}}
{{#subobject:
|Label Page=Carbamide |Label Name=Carbamide11.png
}}