Malaria pathophysiology: Difference between revisions
YazanDaaboul (talk | contribs) |
YazanDaaboul (talk | contribs) |
||
| Line 17: | Line 17: | ||
The bite of Anopheles mosquito allows it to ingest the gametocytes within the red blood cells. In the mosquito's gut, the cells burst and the gametocytes are then released allowing their development into mature gametes. The fusion of male and female gametes forms diploid zygotes and later form oocysts within the mosquito midgut wall.<ref>{{cite web |url=http://www.niaid.nih.gov/topics/malaria/pages/lifecycle.aspx |title= Malaria |date= Apr. 3 2012 |website= National Institute of Allergy and Infectious Diseases|publisher=NIH|accessdate=Jul 24 2014}}</ref> As oocysts continue to grow, they divide and form active haploid forms, the sporozoites. Thousands of sporozoites are produced in each oocyst. The latter bursts after 1-2 weeks and sporozoites travel to the mosquito salivary glands to re-infect humans when the mosquito bites humans and inject the sporozoite into the bloodstream, allowing the cycle of restart.<ref>{{cite web |url=http://www.niaid.nih.gov/topics/malaria/pages/lifecycle.aspx |title= Malaria |date= Apr. 3 2012 |website= National Institute of Allergy and Infectious Diseases|publisher=NIH|accessdate=Jul 24 2014}}</ref> | The bite of Anopheles mosquito allows it to ingest the gametocytes within the red blood cells. In the mosquito's gut, the cells burst and the gametocytes are then released allowing their development into mature gametes. The fusion of male and female gametes forms diploid zygotes and later form oocysts within the mosquito midgut wall.<ref>{{cite web |url=http://www.niaid.nih.gov/topics/malaria/pages/lifecycle.aspx |title= Malaria |date= Apr. 3 2012 |website= National Institute of Allergy and Infectious Diseases|publisher=NIH|accessdate=Jul 24 2014}}</ref> As oocysts continue to grow, they divide and form active haploid forms, the sporozoites. Thousands of sporozoites are produced in each oocyst. The latter bursts after 1-2 weeks and sporozoites travel to the mosquito salivary glands to re-infect humans when the mosquito bites humans and inject the sporozoite into the bloodstream, allowing the cycle of restart.<ref>{{cite web |url=http://www.niaid.nih.gov/topics/malaria/pages/lifecycle.aspx |title= Malaria |date= Apr. 3 2012 |website= National Institute of Allergy and Infectious Diseases|publisher=NIH|accessdate=Jul 24 2014}}</ref> | ||
While parasites generally shift from a sporozoite into a morozoite as they invade red blood cells, some species, such as P. vivax and P. ovale are characterized by their ability to produce hypnozoites, an intermediate stage where the parasite remains dormant for a few months/years before reactivation into merozoites. The hypnozoite stage gives these species the capacity to demonstrate late relapses and long incubation periods.<ref>{{cite journal | author = Cogswell F | title = The hypnozoite and relapse in primate malaria. | url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=358221&blobtype=pdf| journal = Clin Microbiol Rev | volume = 5 | issue = 1 | pages = 26-35 | year = 1992 |id = PMID 1735093}}</ref> | While parasites generally shift from a sporozoite into a morozoite as they invade red blood cells, some species, such as ''P. vivax'' and ''P. ovale'' are characterized by their ability to produce hypnozoites, an intermediate stage where the parasite remains dormant for a few months/years before reactivation into merozoites. The hypnozoite stage gives these species the capacity to demonstrate late relapses and long incubation periods.<ref>{{cite journal | author = Cogswell F | title = The hypnozoite and relapse in primate malaria. | url=http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=358221&blobtype=pdf| journal = Clin Microbiol Rev | volume = 5 | issue = 1 | pages = 26-35 | year = 1992 |id = PMID 1735093}}</ref> | ||
[[Image:MalariacycleBig.jpg|thumb|left|400px|The life cycle of malaria parasites in the human body. The various stages in this process are discussed in the text.]] | [[Image:MalariacycleBig.jpg|thumb|left|400px|The life cycle of malaria parasites in the human body. The various stages in this process are discussed in the text.]] | ||
Revision as of 02:25, 25 July 2014
|
Malaria Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Malaria pathophysiology On the Web |
|
American Roentgen Ray Society Images of Malaria pathophysiology |
|
Risk calculators and risk factors for Malaria pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Malaria in humans develops via two phases: an exoerythrocytic (hepatic) and an erythrocytic phase. When an infected mosquito pierces a person's skin to take a blood meal, sporozoites in the mosquito's saliva enter the bloodstream and migrate to the liver.
Pathophysiology
Malaria is caused by protozoan parasites of the genus Plasmodium (phylumApicomplexa). In humans malaria is caused by P. falciparum, P. malariae, P. ovale, and P. vivax. P. vivax is the most common cause of infection, responsible for about 80 % of all malaria cases. However, P. falciparumis the most important cause of disease, and responsible for about 15% of infections and 90% of deaths.[1]
Life Cycle
The life cycle of Plasmodium parasite starts when the sporozoite, a haploid form of the parasite, is injected into the human bloodstream by the female Anopheles mosquito.[2] The sporozoites then travel in the bloodstream into the liver and invade human hepatocytes. Over 1-2 weeks later, the sporozoites grow and produce thousands of merozoites in each hepatocyte in the exoerythrocytic phase. The merozoite is also a halpoid form of the parasite.[3] While some hepatocytes rupture and release the merozoites within, other parasites remain dormant within the liver.[4] The release of these merozoites from the hepatocytes into the bloodsteam causes the symptoms of malaria. Most importantly, this latency of cell rupture between various hepatocytes and the consequent merozoite release into the bloodstream is responsible for the characteristic periodic fever associated with malaria infections.[5]
As merozoites are released into the bloodstream, they infect erythrocytes (red blood cells). Some merozoites continue the cycle of asexual replication, while others develop into sexual forms, the gametocytes, which involve male and female parasites.[6]
The bite of Anopheles mosquito allows it to ingest the gametocytes within the red blood cells. In the mosquito's gut, the cells burst and the gametocytes are then released allowing their development into mature gametes. The fusion of male and female gametes forms diploid zygotes and later form oocysts within the mosquito midgut wall.[7] As oocysts continue to grow, they divide and form active haploid forms, the sporozoites. Thousands of sporozoites are produced in each oocyst. The latter bursts after 1-2 weeks and sporozoites travel to the mosquito salivary glands to re-infect humans when the mosquito bites humans and inject the sporozoite into the bloodstream, allowing the cycle of restart.[8]
While parasites generally shift from a sporozoite into a morozoite as they invade red blood cells, some species, such as P. vivax and P. ovale are characterized by their ability to produce hypnozoites, an intermediate stage where the parasite remains dormant for a few months/years before reactivation into merozoites. The hypnozoite stage gives these species the capacity to demonstrate late relapses and long incubation periods.[9]

Escaping the Immune System
The parasite is relatively protected from attack by the body's immune system because for most of its human life cycle it resides within the liver and red blood cells and is relatively invisible to immune surveillance. However, circulating infected blood cells are destroyed in the spleen. To avoid this fate, the P. falciparum parasite displays adhesive proteins on the surface of the infected blood cells, causing the blood cells to stick to the walls of small blood vessels, thereby sequestering the parasite from passage through the general circulation and the spleen.[10] This "stickiness" is the main factor giving rise to hemorrhagic complications of malaria. High endothelial venules (the smallest branches of the circulatory system) can be blocked by the attachment of masses of these infected red blood cells. The blockage of these vessels causes symptoms such as in placental and cerebral malaria. In cerebral malaria the sequestrated red blood cells can breach the blood brain barrierpossibly leading to coma.[11]
Although the red blood cell surface adhesive proteins (called PfEMP1, for Plasmodium falciparum erythrocyte membrane protein 1) are exposed to the immune system they do not serve as good immune targets because of their extreme diversity; there are at least 60 variations of the protein within a single parasite and perhaps limitless versions within parasite populations.[10] Like a thief changing disguises or a spy with multiple passports, the parasite switches between a broad repertoire of PfEMP1 surface proteins, thus staying one step ahead of the pursuing immune system.
Some merozoites turn into male and female gametocytes. If a mosquito pierces the skin of an infected person, it potentially picks up gametocytes within the blood. Fertilization and sexual recombination of the parasite occurs in the mosquito's gut, thereby defining the mosquito as the definitive host of the disease. New sporozoites develop and travel to the mosquito's salivary gland, completing the cycle. Pregnant women are especially attractive to the mosquitoes,[12] and malaria in pregnant women is an important cause of stillbirths, infant mortality and low birth weight.[13]
Evolution of Malarial Parasite
Malaria is thought to have been the greatest selective pressure on thehuman genome in recent history.[14] This is due to the high levels ofmortality and morbidity caused by malaria, especially the P. falciparum species.
Sickle-cell Disease and Malaria
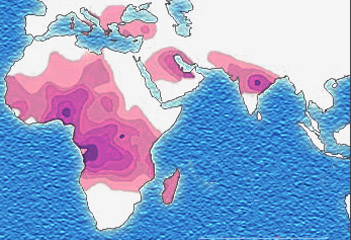
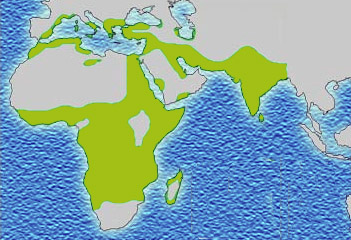
The best-studied influence of the malaria parasite upon the human genome is the blood disease, sickle-cell disease. In sickle-cell disease, there is a mutation in theHBB gene, which encodes the beta globin subunit of haemoglobin. The normal allele encodes a glutamate at position six of the beta globin protein, while the sickle-cell allele encodes a valine.[15] This change from a hydrophilic to a hydrophobic amino acid encourages binding between haemoglobin molecules, with polymerization of haemoglobin deforming red blood cells into a "sickle" shape.[15] Such deformed cells are cleared rapidly from the blood, mainly in the spleen, for destruction and recycling.
In the merozoite stage of its life cycle, the malaria parasite lives inside red blood cells, and its metabolism changes the internal chemistry of the red blood cell. Infected cells normally survive until the parasite reproduces, but if the red cell contains a mixture of sickle and normal haemoglobin, it is likely to become deformed and be destroyed before the daughter parasites emerge. Thus, individuals heterozygous for the mutated allele, known as sickle-cell trait, may have a low and usually unimportant level of anaemia, but also have a greatly reduced chance of serious malaria infection. This is a classic example of heterozygote advantage.[15]
Individuals homozygous for the mutation have full sickle-cell disease and in underdeveloped societies rarely live beyond adolescence. However, in populations where malaria is endemic, the frequency of sickle-cell genes is around 10%. The existence of four haplotypes of sickle-type hemoglobin suggests that this mutation emerged independently at least four times in malaria-endemic areas, further demonstrating its evolutionary advantage in such affected regions.[16]There are also other mutations of the HBB gene that produce haemoglobin molecules capable of conferring similar resistance to malaria infection. These mutations produce haemoglobin types HbE and HbC, which are common in Southeast Asia and Western Africa, respectively.[17]
Thalassaemias
Another well-documented set of mutations found in the human genome associated with malaria are those involved in causing blood disorders known as thalassaemias. Studies in Sardinia and Papua New Guinea have found that the gene frequency ofβ-thalassaemias is related to the level of malarial endemicity in a given population. A study on more than 500 children in Liberia found that those with β-thalassaemia had a 50% decreased chance of getting clinical malaria. Similar studies have found links between gene frequency and malaria endemicity in the α+ form of α-thalassaemia. Presumably these genes have also been selected in the course of human evolution.
Duffy Antigens
The Duffy antigens are antigens expressed on red blood cells and other cells in the body acting as a chemokine receptor. The expression of Duffy antigens on blood cells is encoded by Fy genes (Fya, Fyb, Fyc etc.). Plasmodium vivax malaria uses the Duffy antigen to enter blood cells. However, it is possible to express no Duffy antigen on red blood cells (Fy-/Fy-). This genotype confers complete resistance toP. vivax infection. The genotype is very rare in European, Asian and American populations, but is found in almost all of the indigenous population of West and Central Africa.[18] This is thought to be due to very high exposure to P. vivax in Africa in the last few thousand years.
G6PD
Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme which normally protects from the effects of oxidative stress in red blood cells. However, a genetic deficiency in this enzyme results in increased protection against severe malaria.
HLA and Interleukin-4
HLA-B53 is associated with low risk of severe malaria. ThisMHC class I molecule presents liver stage andsporozoite antigens to T-Cells. Interleukin-4, encoded by IL4, is produced by activated T cells and promotes proliferation and differentiation of antibody-producing B cells. A study of the Fulani of Burkina Faso, who have both fewer malaria attacks and higher levels of antimalarial antibodies than do neighboring ethnic groups, found that the IL4-524 T allele was associated with elevated antibody levels against malaria antigens, which raises the possibility that this might be a factor in increased resistance to malaria.[19]
Chronic Malaria
Chronic malaria is seen in both P. vivax and P. ovale, but not in P. falciparum. Here, the disease can relapse months or years after exposure, due to the presence of latent parasites in the liver. Describing a case of malaria as cured by observing the disappearance of parasites from the bloodstream can therefore be deceptive. The longest incubation period reported for a P. vivax infection is 30 years. Approximately one in five of P. vivax malaria cases in temperate areas involve overwintering by hypnozoites (i.e., relapses begin the year after the mosquito bite).[20]
References
- ↑ Mendis K, Sina B, Marchesini P, Carter R (2001). "The neglected burden of Plasmodium vivax malaria" (PDF). Am J Trop Med Hyg. 64 (1-2 Suppl): 97–106. PMID 11425182.
- ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ "Malaria". National Institute of Allergy and Infectious Diseases. NIH. Apr. 3 2012. Retrieved Jul 24 2014. Check date values in:
|accessdate=, |date=(help) - ↑ Cogswell F (1992). "The hypnozoite and relapse in primate malaria". Clin Microbiol Rev. 5 (1): 26–35. PMID 1735093.
- ↑ 10.0 10.1 Chen Q, Schlichtherle M, Wahlgren M (2000). "Molecular aspects of severe malaria". Clin Microbiol Rev. 13 (3): 439–50. PMID 10885986.
- ↑ Adams S, Brown H, Turner G (2002). "Breaking down the blood-brain barrier: signaling a path to cerebral malaria?". Trends Parasitol. 18 (8): 360–6. PMID 12377286.
- ↑ Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G (2000). "Effect of pregnancy on exposure to malaria mosquitoes". Lancet. 355 (9219): 1972. PMID 10859048.
- ↑ van Geertruyden J, Thomas F, Erhart A, D'Alessandro U (2004). "The contribution of malaria in pregnancy to perinatal mortality". Am J Trop Med Hyg. 71 (2 Suppl): 35–40. PMID 15331817.
- ↑ Kwiatkowski, DP (2005). "How Malaria Has Affected the Human Genome and What Human Genetics Can Teach Us about Malaria". Am J Hum Genet. 77: 171–92. PMID 16001361.
- ↑ 15.0 15.1 15.2 Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW; et al. (2005). "Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases". J Infect Dis. 192 (1): 178–86. doi:10.1086/430744. PMC 3545189. PMID 15942909.
- ↑ BEUTLER E, DERN RJ, FLANAGAN CL (1955). "Effect of sickle-cell trait on resistance to malaria". Br Med J. 1 (4923): 1189–91. PMC 2062141. PMID 14363831.
- ↑ Williams TN, Mwangi TW, Roberts DJ, Alexander ND, Weatherall DJ, Wambua S; et al. (2005). "An immune basis for malaria protection by the sickle cell trait". PLoS Med. 2 (5): e128. doi:10.1371/journal.pmed.0020128. PMC 1140945. PMID 15916466.
- ↑ Carter R, Mendis KN (2002). "Evolutionary and historical aspects of the burden of malaria". Clin. Microbiol. Rev. 15 (4): 564–94. PMID 12364370.
- ↑ Verra F, Luoni G, Calissano C, Troye-Blomberg M, Perlmann P, Perlmann H, Arcà B, Sirima B, Konaté A, Coluzzi M, Kwiatkowski D, Modiano D (2004). "IL4-589C/T polymorphism and IgE levels in severe malaria". Acta Trop. 90 (2): 205–9. PMID 15177147.
- ↑ Adak T, Sharma V, Orlov V (1998). "Studies on the Plasmodium vivax relapse pattern in Delhi, India". Am J Trop Med Hyg. 59 (1): 175–9. PMID 9684649.