Caffeine citrate (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 76: | Line 76: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* [[Oral]] | ||
* Intravenous | * [[Intravenous]] | ||
|monitoring=* Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity. Serious toxicity has been associated with serum levels greater than 50 mg/L. | |monitoring=* Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity. Serious toxicity has been associated with serum levels greater than 50 mg/L. | ||
* All preterm infants, patients being treated with caffeine citrate should be carefully monitored for the development of [[necrotizing enterocolitis]]. | * All preterm infants, patients being treated with caffeine citrate should be carefully monitored for the development of [[necrotizing enterocolitis]]. | ||
Revision as of 20:04, 9 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Caffeine citrate (injection) is a methylxanthine that is FDA approved for the treatment of apnea of prematurity in infants between 28 and < 33 weeks gestational age. Common adverse reactions include irritability, feeding problem symptom.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Caffeine citrate injection is indicated for the short-term treatment of apnea of prematurity in infants between 28 and < 33 weeks gestational age.
- Prior to initiation of caffeine citrate injection, baseline serum levels of caffeine should be measured in infants previously treated with theophylline, since preterm infants metabolize theophylline to caffeine. Likewise, baseline serum levels of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery, since caffeine readily crosses the placenta.
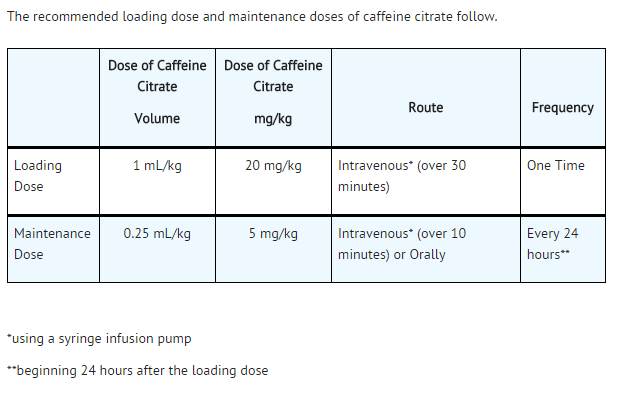
- The recommended loading dose and maintenance doses of caffeine citrate follow.
- NOTE THAT THE DOSE OF CAFFEINE BASE IS ONE-HALF THE DOSE WHEN EXPRESSED AS CAFFEINE CITRATE (e.g., 20 mg of caffeine citrate is equivalent to 10 mg of caffeine base).
- Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity. Serious toxicity has been associated with serum levels greater than 50 mg/L.
- Caffeine citrate injection should be inspected visually for particulate matter and discoloration prior to administration. Vials containing discolored solution or visible particulate matter should be discarded.
Drug Compatibility
- To test for drug compatibility with common intravenous solutions or medications, 20 mL of caffeine citrate injection were combined with 20 mL of a solution or medication, with the exception of an Intralipid® admixture, which was combined as 80 mL/80 mL. The physical appearance of the combined solutions was evaluated for precipitation. The admixtures were mixed for 10 minutes and then assayed for caffeine. The admixtures were then continually mixed for 24 hours, with further sampling for caffeine assays at 2, 4, 8, and 24 hours.
- Based on this testing, caffeine citrate injection, 60 mg/3 mL is chemically stable for 24 hours at room temperature when combined with the following test products.
- Dextrose Injection, USP 5%
- 50% Dextrose Injection USP
- Intralipid® 20% IV Fat Emulsion
- Aminosyn® 8.5% Crystalline Amino Acid Solution
- Dopamine HCI Injection, USP 40 mg/mL diluted to 0.6 mg/mL with Dextrose Injection, USP 5%
- Calcium Gluconate Injection, USP 10% (0.465 mEq/Ca+2/mL)
- Heparin Sodium Injection, USP 1,000 units/mL diluted to 1 unit/mL with Dextrose Injection, USP 5%
- Fentanyl Citrate Injection, USP 50 mcg/mL diluted to 10 mcg/mL with Dextrose Injection, USP 5%
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Caffeine citrate (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Caffeine citrate (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Caffeine citrate (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Caffeine citrate (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Caffeine citrate (injection) in pediatric patients.
Contraindications
- Caffeine citrate is contraindicated in patients who have demonstrated hypersensitivity to any of its components.
Warnings
- During the double-blind, placebo-controlled clinical trial, six cases of necrotizing enterocolitis developed among the 85 infants studied (caffeine=46, placebo=39), with three cases resulting in death. Five of the six patients with necrotizing enterocolitis were randomized to or had been exposed to caffeine citrate.
- Reports in the published literature have raised a question regarding the possible association between the use of methylxanthines and development of necrotizing enterocolitis, although a causal relationship between methylxanthine use and necrotizing enterocolitis has not been established. Therefore, as with all preterm infants, patients being treated with caffeine citrate should be carefully monitored for the development of necrotizing enterocolitis.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Caffeine citrate (injection) Clinical Trials Experience in the drug label.
Postmarketing Experience
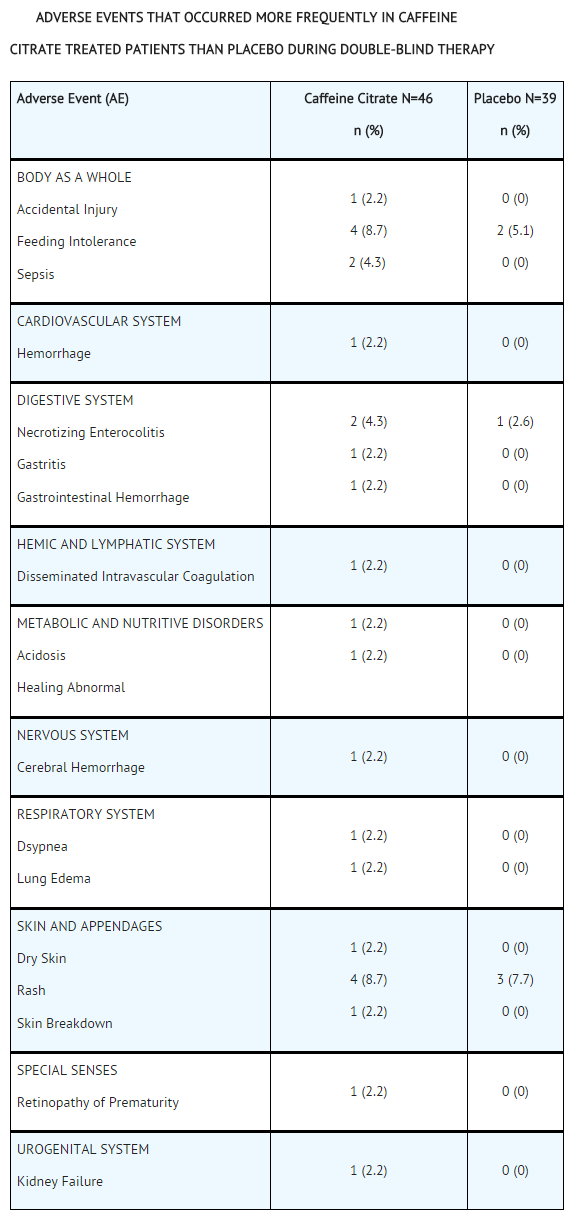
- Overall, the reported number of adverse events in the double-blind period of the controlled trial was similar for the caffeine citrate and placebo groups. The following table shows adverse events that occurred in the double-blind period of the controlled trial and that were more frequent in caffeine citrate treated patients than placebo.
- Overall, the reported number of adverse events in the double-blind period of the controlled trial was similar for the caffeine citrate and placebo groups. The following table shows adverse events that occurred in the double-blind period of the controlled trial and that were more frequent in caffeine citrate treated patients than placebo.
Drug Interactions
There is limited information regarding Caffeine citrate (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Concern for the teratogenicity of caffeine is not relevant when administered to infants. In studies performed in adult animals, caffeine (as caffeine base) administered to pregnant mice as sustained release pellets at 50 mg/kg (less than the maximum recommended intravenous loading dose for infants on a mg/m2 basis), during the period of organogenesis, caused a low incidence of cleft palate and exencephaly in the fetuses. There are no adequate and well-controlled studies in pregnant women.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Caffeine citrate (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Caffeine citrate (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Caffeine citrate (injection) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Caffeine citrate (injection) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Caffeine citrate (injection) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Caffeine citrate (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Caffeine citrate (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Caffeine citrate (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Caffeine citrate (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Caffeine citrate (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Caffeine citrate (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Serum concentrations of caffeine may need to be monitored periodically throughout treatment to avoid toxicity. Serious toxicity has been associated with serum levels greater than 50 mg/L.
- All preterm infants, patients being treated with caffeine citrate should be carefully monitored for the development of necrotizing enterocolitis.
IV Compatibility
There is limited information regarding IV Compatibility of Caffeine citrate (injection) in the drug label.
Overdosage
- Following overdose, serum caffeine levels have ranged from approximately 24 mg/L (a post marketing spontaneous case report in which an infant exhibited irritability, poor feeding and insomnia) to 350 mg/L. Serious toxicity has been associated with serum levels greater than 50 mg/L.
- Signs and symptoms reported in the literature after caffeine overdose in preterm infants include fever, tachypnea, jitteriness, insomnia, fine tremor of the extremities, hypertonia, opisthotonos, tonic-clonic movements, nonpurposeful jaw and lip movements, vomiting, hyperglycemia, elevated blood urea nitrogen, and elevated total leukocyte concentration. Seizures have also been reported in cases of overdose. One case of caffeine overdose complicated by development of intraventricular hemorrhage and long-term neurological sequelae has been reported. Another case of caffeine citrate overdose (from New Zealand) of an estimated 600 mg caffeine citrate (approximately 322 mg/kg) administered over 40 minutes was complicated by tachycardia, ST depression, respiratory distress, heart failure, gastric distention, acidosis and a severe extravasation burn with tissue necrosis at the peripheral intravenous injection site. No deaths associated with caffeine overdose have been reported in preterm infants.
- Treatment of caffeine overdose is primarily symptomatic and supportive. Caffeine levels have been shown to decrease after exchange transfusions. Convulsions may be treated with intravenous administration of diazepam or a barbiturate such as pentobarbital sodium.
Pharmacology
There is limited information regarding Caffeine citrate (injection) Pharmacology in the drug label.
Mechanism of Action
- Caffeine is structurally related to other methylxanthines, theophylline and theobromine. It is a bronchial smooth muscle relaxant, a CNS stimulant, a cardiac muscle stimulant and a diuretic.
- Although the mechanism of action of caffeine in apnea of prematurity is not known, several mechanisms have been hypothesized. These include: (1) stimulation of the respiratory center, (2) increased minute ventilation, (3) decreased threshold to hypercapnia, (4) increased response to hypercapnia, (5) increased skeletal muscle tone, (6) decreased diaphragmatic fatigue, (7) increased metabolic rate, and (8) increased oxygen consumption.
- Most of these effects have been attributed to antagonism of adenosine receptors, both A1 and A2 subtypes, by caffeine, which has been demonstrated in receptor binding assays and observed at concentrations approximating those achieved therapeutically.
Structure
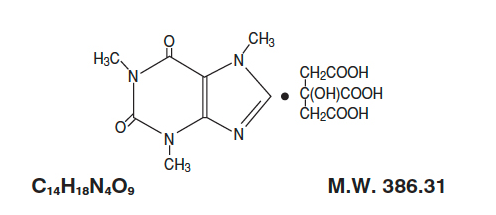
- Caffeine citrate injection, USP for intravenous administration is a clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solution adjusted to pH 4.7. Each mL contains 20 mg caffeine citrate (equivalent to 10 mg of caffeine base) prepared in solution by the addition of 10 mg caffeine anhydrous to 5 mg citric acid monohydrate, 8.3 mg sodium citrate dihydrate and Water for Injection.
- Caffeine, a central nervous system stimulant, is an odorless white crystalline powder or granule, with a bitter taste. It is sparingly soluble in water and ethanol at room temperature. The chemical name of caffeine is 3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione. In the presence of citric acid it forms caffeine citrate salt in solution. The structural formula and molecular weight of caffeine citrate follows.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Caffeine citrate (injection) in the drug label.
Pharmacokinetics
Absorption
- After oral administration of 10 mg caffeine base/kg to preterm neonates, the peak plasma level (Cmax) for caffeine ranged from 6 to 10 mg/L and the mean time to reach peak concentration (Tmax) ranged from 30 minutes to 2 hours. The Tmax was not affected by formula feeding. The absolute bioavailability, however, was not fully examined in preterm neonates.
Distribution
- Caffeine is rapidly distributed into the brain. Caffeine levels in the cerebrospinal fluid of preterm neonates approximate their plasma levels. The mean volume of distribution of caffeine in infants (0.8 to 0.9 L/kg) is slightly higher than that in adults (0.6 L/kg). Plasma protein binding data are not available for neonates or infants. In adults, the mean plasma protein binding in vitro is reported to be approximately 36%.
Metabolism
- Hepatic cytochrome P450 1A2 (CYP1A2) is involved in caffeine biotransformation. Caffeine metabolism in preterm neonates is limited due to their immature hepatic enzyme systems.
- Interconversion between caffeine and theophylline has been reported in preterm neonates; caffeine levels are approximately 25% of theophylline levels after theophylline administration and approximately 3 to 8% of caffeine administered would be expected to convert to theophylline.
Elimination
- In young infants, the elimination of caffeine is much slower than that in adults due to immature hepatic and/or renal function. Mean half-life (T1/2) and fraction excreted unchanged in urine (Ae) of caffeine in infants have been shown to be inversely related to gestational/postconceptual age. In neonates, the T1/2 is approximately 3 to 4 days and the Ae is approximately 86% (within 6 days). By 9 months of age, the metabolism of caffeine approximates that seen in adults (T1/2 = 5 hours and Ae = 1%).
Special Populations
- Studies examining the pharmacokinetics of caffeine in neonates with hepatic or renal insufficiency have not been conducted. Caffeine citrate should be administered with caution in preterm neonates with impaired renal or hepatic function. Serum concentrations of caffeine should be monitored and dose administration of caffeine citrate should be adjusted to avoid toxicity in this population.
Clinical Studies
- One multicenter, randomized, double-blind trial compared caffeine citrate to placebo in eighty-five (85) preterm infants (gestational age 28 to <33 weeks) with apnea of prematurity. Apnea of prematurity was defined as having at least 6 apnea episodes of greater than 20 seconds duration in a 24 hour period with no other identifiable cause of apnea. A 1 mL/kg (20 mg/kg caffeine citrate providing 10 mg/kg as caffeine base) loading dose of caffeine citrate was administered intravenously, followed by a 0.25 mL/kg (5 mg/kg caffeine citrate providing 2.5 mg/kg of caffeine base) daily maintenance dose administered either intravenously or orally (generally through a feeding tube). The duration of treatment in this study was limited to 10 to 12 days. The protocol allowed infants to be “rescued” with open-label caffeine citrate treatment if their apnea remained uncontrolled during the double-blind phase of the trial.
- The percentage of patients without apnea on day 2 of treatment (24 to 48 hours after the loading dose) was significantly greater with caffeine citrate than placebo. The following table summarizes the clinically relevant endpoints evaluated in this study:
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Caffeine citrate (injection) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Caffeine citrate (injection) in the drug label.
How Supplied
- Caffeine citrate injection, USP is available as a clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solution in colorless glass vials.
- The vials contain 3 mL of solution at a concentration of 20 mg/mL caffeine citrate (60 mg/vial) equivalent to 10 mg/mL caffeine base (30 mg/vial).
- Caffeine Citrate Injection, USP
Storage
- Store at 20° to 25°C (68° to 77°F)[see USP Controlled Room Temperature].
- Preservative Free. For single use only. Discard unused portion.
- This container closure is not made with natural rubber latex.
- The brand names mentioned in this document are trademarks of their respective owners.
Images
Drug Images
{{#ask: Page Name::Caffeine citrate (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Caffeine citrate (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Caffeine citrate (injection) in the drug label.
Precautions with Alcohol
- Alcohol-Caffeine citrate (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CAFFEINE CITRATE ®[1]
Look-Alike Drug Names
There is limited information regarding Caffeine citrate (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Caffeine citrate (injection) |Label Name=Caffine 06 Display.jpg
}}
{{#subobject:
|Label Page=Caffeine citrate (injection) |Label Name=Caffine 07 Display.jpg
}}
{{#subobject:
|Label Page=Caffeine citrate (injection) |Label Name=DailyMed - CAFFEINE CITRATE- caffeine citrate injection, solution .png
}}