Finerenone: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
Table 1 summarizes the Recommended Starting Dosage. | Table 1 summarizes the Recommended Starting Dosage. | ||
[[Image:Finerenone Table 1 Dosage.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|offLabelAdultGuideSupport=There is limited information regarding ''Off-Label Guideline-Supported Use'' of Finerenone in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding ''Off-Label Guideline-Supported Use'' of Finerenone in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding ''Off-Label Non–Guideline-Supported Use'' of Finerenone in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding ''Off-Label Non–Guideline-Supported Use'' of Finerenone in adult patients. | ||
| Line 42: | Line 42: | ||
Table 3 summarizes the Adverse Reactions in the Phase 3 Study FIDELIO-DKD. | Table 3 summarizes the Adverse Reactions in the Phase 3 Study FIDELIO-DKD. | ||
[[Image:Finerenone Table 3 Adverse Reactions.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
| Line 77: | Line 77: | ||
Table 2 summarizes Dose Adjustment Based on Current Serum Potassium Concentration and Current Dose. | Table 2 summarizes Dose Adjustment Based on Current Serum Potassium Concentration and Current Dose. | ||
[[Image:Finerenone Table 2 Dosage Adjustments.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|overdose=*Reduce or stop Finerenone treatment if overdose is suspected. | |overdose=*Reduce or stop Finerenone treatment if overdose is suspected. | ||
*Hyperkalemia is the most likely manifestation of an overdose. | *Hyperkalemia is the most likely manifestation of an overdose. | ||
*Removal of Finerenone through hemodialysis is unlikely. | *Removal of Finerenone through hemodialysis is unlikely. | ||
|drugBox={{drugbox2 | |||
| drug_name = | |||
| image = Finerenone Structure 2.png | |||
| alt = | |||
| caption = | |||
<!-- Clinical data --> | |||
| pronounce = | |||
| tradename = Kerendia | |||
| Drugs.com = | |||
| MedlinePlus = | |||
| licence_EU = <!-- EMA uses INN (or special INN_EMA) --> | |||
| DailyMedID = Finerenone | |||
| licence_US = <!-- FDA may use generic or brand name (generic name preferred) --> | |||
| pregnancy_AU = D | |||
| pregnancy_AU_comment = <ref>{{cite web | title=Updates to the Prescribing Medicines in Pregnancy database | website=Therapeutic Goods Administration (TGA) | date=12 May 2022 | url=https://www.tga.gov.au/updates-prescribing-medicines-pregnancy-database | access-date=13 May 2022}}</ref><ref name="Kerendia APMDS" /> | |||
| pregnancy_category= | |||
| routes_of_administration = [[Oral administration|By mouth]] | |||
| class = [[Potassium-sparing diuretic]] | |||
| ATC_prefix = C03 | |||
| ATC_suffix = DA05 | |||
| ATC_supplemental = | |||
<!-- Legal status --> | |||
| legal_AU = S4 | |||
| legal_AU_comment = <ref name="Kerendia APMDS">{{cite web | title=Kerendia APMDS | website=Therapeutic Goods Administration (TGA) | date=9 December 2021 | url=https://www.tga.gov.au/apm-summary/kerendia | access-date=12 June 2022}}</ref><ref>{{cite web | title=AusPAR: Finerenone | website=Therapeutic Goods Administration (TGA) | date=31 May 2022 | url=https://www.tga.gov.au/auspar/auspar-finerenone | access-date=12 June 2022}}</ref> | |||
| legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F --> | |||
| legal_BR_comment = | |||
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_CA_comment = | |||
| legal_DE = <!-- Anlage I, II, III or Unscheduled --> | |||
| legal_DE_comment = | |||
| legal_NZ = <!-- Class A, B, C --> | |||
| legal_NZ_comment = | |||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM / Class A, B, C --> | |||
| legal_UK_comment = | |||
| legal_US = Rx-only | |||
| legal_US_comment = <ref name="Kerendia FDA label">{{cite web | title=Kerendia- finerenone tablet, film coated | website=DailyMed | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fc726765-5d5a-4d6e-b037-b847bda9fb7c | access-date=20 August 2021}}</ref><ref name="FDA Kerendia" /> | |||
| legal_EU = Rx-only | |||
| legal_EU_comment = <ref name="Kerendia EPAR">{{cite web | title=Kerendia EPAR | website=[[European Medicines Agency]] (EMA) | date=14 December 2021 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/kerendia | access-date=11 March 2022}} Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.</ref> | |||
| legal_UN = <!-- N I, II, III, IV / P I, II, III, IV --> | |||
| legal_UN_comment = | |||
| legal_status = <!-- For countries not listed above --> | |||
<!-- Pharmacokinetic data --> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| metabolites = | |||
| onset = | |||
| elimination_half-life = | |||
| duration_of_action = | |||
| excretion = | |||
<!-- Identifiers --> | |||
| CAS_number = 1050477-31-0 | |||
| CAS_supplemental = | |||
| PubChem = 60150535 | |||
| IUPHAR_ligand = | |||
| DrugBank = DB16165 | |||
| ChemSpiderID = 28669387 | |||
| UNII = DE2O63YV8R | |||
| KEGG = D10633 | |||
| ChEBI = | |||
| ChEMBL = 2181927 | |||
| NIAID_ChemDB = | |||
| PDB_ligand = | |||
| synonyms = BAY 94-8862 | |||
<!-- Chemical and physical data --> | |||
| IUPAC_name = (4''S'')-4-(4-Cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide | |||
| C=21 | H=22 | N=4 | O=3 | |||
| SMILES = NC(=O)C1=C(C)Nc2c(C)cnc(OCC)c2[C@@H]1c3ccc(C#N)cc3OC | |||
| StdInChI = 1S/C21H22N4O3/c1-5-28-21-18-17(14-7-6-13(9-22)8-15(14)27-4)16(20(23)26)12(3)25-19(18)11(2)10-24-21/h6-8,10,17,25H,5H2,1-4H3,(H2,23,26)/t17-/m1/s1 | |||
| StdInChI_comment = | |||
| StdInChIKey = BTBHLEZXCOBLCY-QGZVFWFLSA-N | |||
| density = | |||
| density_notes = | |||
| melting_point = | |||
| melting_high = | |||
| melting_notes = | |||
| boiling_point = | |||
| boiling_notes = | |||
| solubility = | |||
| sol_units = | |||
| specific_rotation = | |||
}} | |||
|mechAction=*Finerenone is a nonsteroidal, selective antagonist of the mineralocorticoid receptor. | |mechAction=*Finerenone is a nonsteroidal, selective antagonist of the mineralocorticoid receptor. | ||
*MR mediated sodium reabsorption is blocked by Finerenone. | *MR mediated sodium reabsorption is blocked by Finerenone. | ||
| Line 88: | Line 175: | ||
*Finerenone has a molecular weight of 378.43 g/mol. | *Finerenone has a molecular weight of 378.43 g/mol. | ||
[[Image:Finerenone Structure.png|thumb|none|300px|This image is provided by the National Library of Medicine.]] | |||
|PD=*In clinical studies, mean systolic blood pressure decreased by 3 mmHg in patients receiving Finerenone. | |PD=*In clinical studies, mean systolic blood pressure decreased by 3 mmHg in patients receiving Finerenone. | ||
*In clinical studies, mean diastolic blood pressure decreased by 1-2 mmHg in patients receiving Finerenone, but stabilized after a month. | *In clinical studies, mean diastolic blood pressure decreased by 1-2 mmHg in patients receiving Finerenone, but stabilized after a month. | ||
| Line 169: | Line 256: | ||
Table 4 summarizes the Primary and Secondary Time-to-Event Endpoints. | Table 4 summarizes the Primary and Secondary Time-to-Event Endpoints. | ||
[[Image:Finerenone Table 4 Clinical Studies.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Figure 1 shows the Time to first occurrence of kidney failure, sustained decline in eGFR ≥40% from baseline, or renal death in the FIDELIO-DKD study. | Figure 1 shows the Time to first occurrence of kidney failure, sustained decline in eGFR ≥40% from baseline, or renal death in the FIDELIO-DKD study. | ||
[[Image:Finerenone Figure 1 Clincal Studies.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Figure 2 shows the Time to first occurrence of CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for heart failure in the FIDELIO-DKD study. | Figure 2 shows the Time to first occurrence of CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for heart failure in the FIDELIO-DKD study. | ||
[[Image:Finerenone Figure 2 Clinical Studies.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|howSupplied=*Finerenone is either supplied as 10 mg or 20 mg. | |howSupplied=*Finerenone is either supplied as 10 mg or 20 mg. | ||
*10 mg of Finerenone tablets are pink oblong. | *10 mg of Finerenone tablets are pink oblong. | ||
| Line 185: | Line 272: | ||
*Either dosage of Finerenone can be given in 30 or 90 tablet bottles. | *Either dosage of Finerenone can be given in 30 or 90 tablet bottles. | ||
|storage=*Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. | |storage=*Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. | ||
|packLabel=[[Image:Finerenone Drug Label (10 mg).png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[Image:Finerenone Drug Label (20 mg).png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[Image:Finerenone Drug Info.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[Image:Finerenone Drug Info Pt.2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[Image:Finerenone Drug Info Pt.3.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=*Monitor patients serum potassium levels at baseline and during Finerenone treatment. | |fdaPatientInfo=*Monitor patients serum potassium levels at baseline and during Finerenone treatment. | ||
*Advise patients using potassium supplements or salt substitutes containing potassium to consult with their medical provider. | *Advise patients using potassium supplements or salt substitutes containing potassium to consult with their medical provider. | ||
Latest revision as of 00:09, 12 September 2022
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tejasvi Aryaputra
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Finerenone is a mineralocorticoid receptor antagonist that is FDA approved for the prophylaxis of the risk of kidney and heart complications in chronic kidney disease associated with type 2 diabetes. Common adverse reactions include hypotension, hyperkalemia, and hyponatremia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
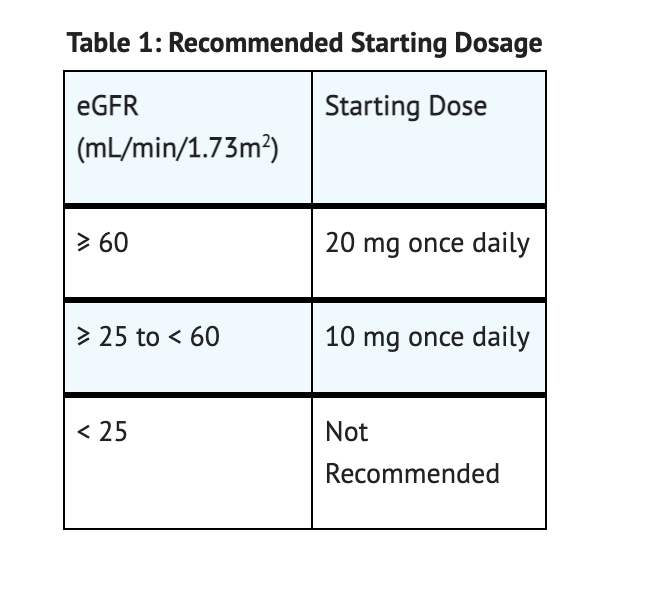
Recommended Starting Dosage
Table 1 summarizes the Recommended Starting Dosage.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Finerenone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Finerenone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Finerenone FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Finerenone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Finerenone in pediatric patients.
Contraindications
- Patients receiving concomitant treatment with strong CYP3A4 inhibitors.
- Patients with adrenal insufficiency.
Warnings
Hyperkalemia
- Patients taking Finerenone may experience hyperkalemia.
- Patients with a decrease in kidney function are more likely to experience hyperkalemia.
- Monitor patients eGFR and serum potassium levels before and during Finerenone treatment.
- Advise patients to avoid Finerenone if they have serum potassium level that is > 5.0 mEq/L.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
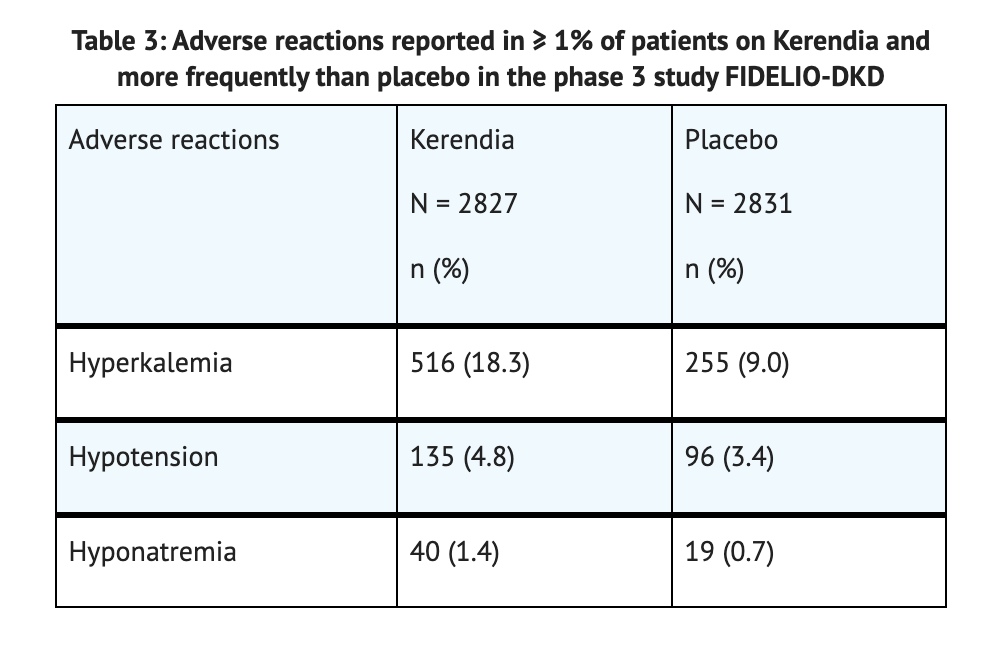
Phase 3 study FIDELIO-DKD
- A randomized, double-blind, placebo-controlled, multicenter pivotal phase 3 study was conducted on 5658 patients to test the safety of Finerenone.
- 2831 patients were part of the placebo group while 2827 patients were part of the Finerenone group.
- 2.2 years is the mean duration of Finerenone treatment.
- 32% of patients in the Finerenone group experienced serious adverse reactions.
- 34% of patients in the placebo group experienced serious adverse reactions.
- 7% of patients in the Finerenone group experienced permeant discontinuation due to serious adverse reactions.
- 6% of patients in the placebo group experienced permeant discontinuation due to serious adverse reactions.
- 2.3% of patients in the Finerenone group experienced hyperkalemia which led to permanent discontinuation.
- 0.9% of patients in the placebo group experienced hyperkalemia which led to permanent discontinuation.
- Hyperkalemia was the most common adverse reaction reported in the study.
Table 3 summarizes the Adverse Reactions in the Phase 3 Study FIDELIO-DKD.
Laboratory Test
- Initial small decrease in estimated GFR may occur in patients taking Finerenone.
- Studies show that the decrease in estimated GFR is reversible.
Postmarketing Experience
There is limited information regarding Finerenone Postmarketing Experience in the drug label.
Drug Interactions
Effects of other drugs on Finerenone
Strong CYP3A4 Inhibitors:
- Finerenone is a CYP3A4 substrate.
- Exposure of Finerenone may increase with concomitant use of a strong CYP3A4 inhibitor.
- The concomitant use of a strong CYP3A4 inhibitor and Finerenone is contraindicated.
- Grapefruit and grapefruit juice should be avoided when taking Finerenone.
Moderate and Weak CYP3A4 Inhibitors:
- Exposure of Finerenone may increase with concomitant use of a moderate or weak CYP3A4 inhibitor.
- Monitor serum potassium levels during treatment.
Strong and Moderate CYP3A4 Inducers:
- Exposure of Finerenone may decrease with concomitant use of a strong or moderate CYP3A4 inducer.
- Advise patients to avoid concomitant use of a strong or moderate CYP3A4 inducer and Finerenone.
Drugs That Affect Serum Potassium
- Monitor serum potassium levels more frequently in patients receiving concomitant therapy with either drugs or supplements that increase serum potassium.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
No studies have been conducted to look at if Finerenone causes major birth defects, miscarriage or adverse maternal or fetal outcomes in humans. In animal studies, developmental toxicity at exposures about 4 times than those in humans when looking at Finerenone. Rat studies show a reduction in placental weights, increase of visceral and skeletal variations, and signs of fetal toxicity when given Finerenone.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Finerenone in women who are pregnant.
Labor and Delivery
No data has been conducted on nursing in human when taking Finerenone. In animal studies, lower pup weight and increased pup mortality was seen when rats were given Finerenone. Studies have shown that Finerenone is found in rat milk which could indicate that Finerenone could be found in human milk.
Nursing Mothers
There is no FDA guidance on the use of Finerenone in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Finerenone in pediatric settings.
Geriatic Use
When looking into the safety and efficacy of Finerenone, there were no indications that there are differences between older and younger patients in clinical studies. No changes of dosage is required between older and younger patients.
Gender
There is no FDA guidance on the use of Finerenone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Finerenone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Finerenone in patients with renal impairment.
Hepatic Impairment
Patients with severe hepatic impairment should avoid Finerenone use. Patients with mild or moderate hepatic impairment does not need a dosage adjustment. Monitor serum levels carefully in patients with moderate hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Finerenone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Finerenone in patients who are immunocompromised.
Administration and Monitoring
Administration
Missed doses
- If missed on the same day, advise patients to take Finerenone as soon as possible.
- If a patient cannot make up missed dose, skip dosage and continue the next day as scheduled.
Monitoring
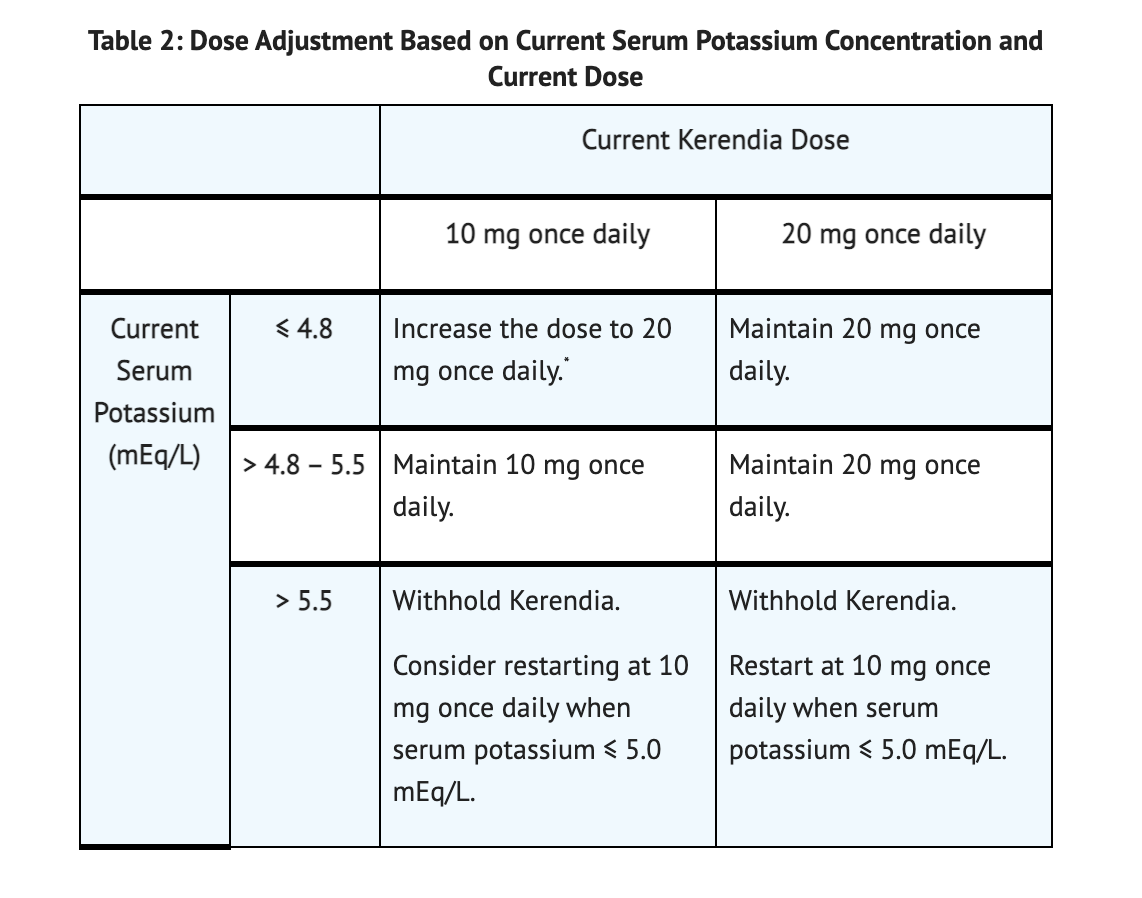
Monitoring and Dose Adjustment
Table 2 summarizes Dose Adjustment Based on Current Serum Potassium Concentration and Current Dose.
IV Compatibility
There is limited information regarding the compatibility of Finerenone and IV administrations.
Overdosage
- Reduce or stop Finerenone treatment if overdose is suspected.
- Hyperkalemia is the most likely manifestation of an overdose.
- Removal of Finerenone through hemodialysis is unlikely.
Pharmacology
| |
Finerenone
| |
| Systematic (IUPAC) name | |
| (4S)-4-(4-Cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide | |
| Identifiers | |
| CAS number | |
| ATC code | C03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | BAY 94-8862 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
D(AU) |
| Legal status |
Prescription Only (S4)(AU) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth |
Mechanism of Action
- Finerenone is a nonsteroidal, selective antagonist of the mineralocorticoid receptor.
- MR mediated sodium reabsorption is blocked by Finerenone.
- MR overactivation in both epithelial and non-epithelial tissue is blocked by Finerenone.
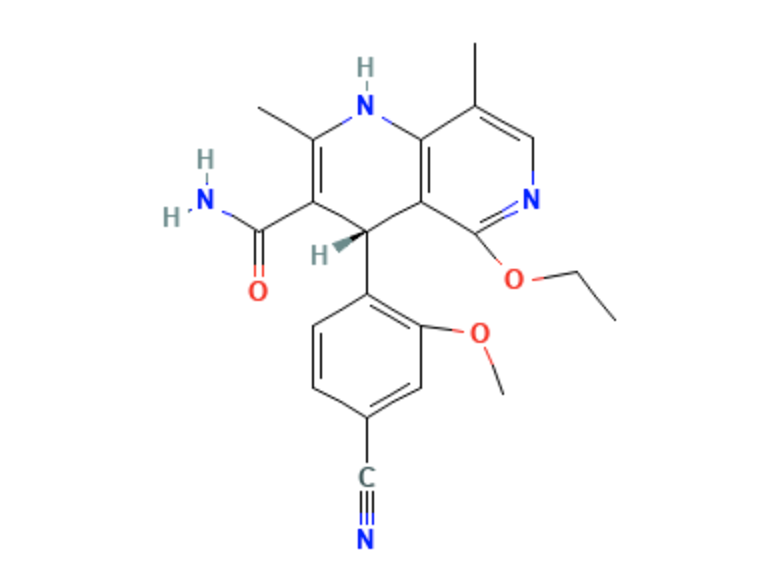
Structure
- Finerenone is a nonsteroidal, selective antagonist of the mineralocorticoid receptor.
- Finerenone has an empirical formula of C21H22N4O3.
- Finerenone has a molecular weight of 378.43 g/mol.

Pharmacodynamics
- In clinical studies, mean systolic blood pressure decreased by 3 mmHg in patients receiving Finerenone.
- In clinical studies, mean diastolic blood pressure decreased by 1-2 mmHg in patients receiving Finerenone, but stabilized after a month.
- In clinical studies, placebo-corrected relative reduction in urinary albumin-to-creatinine ratio in patients randomized to Finerenone was 31% that stabilized after 4 months.
Cardiac Electrophysiology
- Finerenone does not prolong the QT interval at a dose 4 times the maximum approved recommended dose.
Pharmacokinetics
- Finerenone exposure increased proportionally over a dose range of 1.25 to 80 mg.
- 2 days of Finerenone treatment led to steady state.
- After patients were given 20 mg of Finerenone, 160 µg/L is the estimated steady-state geometric mean Cmax,md.
- After patients were given 20 mg of Finerenone, 686 µg.h/L is the estimated steady-state geometric mean AUCT,md.
<b<Absorption
- 44% is the absolute bioavailability of Finerenone.
- 0.5 and 1.25 hours is the time range that Finerenone Cmax occurred after dosing.
Effect of Food
- High fat, high calorie food did not cause clinically significant differences in Finerenone AUC.
Distribution
- 52.6 L is the volume of distribution at steady-state.
- In vitro, 92% is the plasma protein binding of Finerenone to serum albumin.
Elimination
- 2 to 3 hours is terminal half-life of Finerenone.
- 25 L/h is the systemic blood clearance of Finerenone.
Metabolism
- CYP3A4 primarily metabolizes Finerenone to inactive metabolites.
- CYP2C8 metabolizes Finerenone to inactive metabolites.
Excretion
- In feces, 20% of Finerenone was found in which less than 0.2% was found unchanged.
- In urine, 80% of Finerenone was found in which less than 1% was found unchanged.
Specific Populations
- Age, weight, ethnicity, sex, or race effected the pharmacokinetics of Finerenone that were clinically significant.
Renal Impairment
- AUC or Cmax values of Finerenone were not clinically different when comparing patients with eGFR 15 to < 90 mL/min/1.73m2 to patients with eGFR ≥ 90 mL/min/1.73 m2.
Hepatic Impairment
- The exposure of Finerenone is not clinically significant in cirrhotic patients with mild hepatic impairment.
- 38% increase of Finerenone mean AUC was seen in cirrhotic patients with moderate hepatic impairment.
- No change to Cmax was seen in cirrhotic patients with moderate hepatic impairment.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches: Strong CYP3A Inhibitors:
- >400% increase of Finerenone AUC was seen in patients that concomitant used Finerenone and a strong CYP3A4 inhibitor.
Moderate CYP3A Inhibitors:
- 248% increase of Finerenone AUC was seen in patients that concomitant used Finerenone and a moderate CYP3A4 inhibitor.
- 88% increase of Finerenone Cmax was seen in patients that concomitant used Finerenone and a moderate CYP3A4 inhibitor.
Weak CYP3A Inhibitors:
- 21% increase of Finerenone AUC was seen in patients that concomitant used Finerenone and a weak CYP3A4 inhibitor.
Strong or Moderate CYP3A Inducers:
- 80% decrease of Finerenone AUC was seen in patients that concomitant used Finerenone and a moderate CYP3A4 inducer.
- 90% decrease of Finerenone AUC was seen in patients that concomitant used Finerenone and a strong CYP3A4 inducer.
Other Drugs:
- Concomitant use of proton pump inhibitor, aluminium hydroxide and magnesium hydroxide antacid, or a strong CYP2C8 inhibitor with Finerenone had no clinically significant effect on the pharmacokinetics of Finerenone.
- Concomitant use of a P-gp substrate or CYP2C9 substrate with Finerenone had no clinically significant effect on the pharmacokinetics of Finerenone.
- Concomitant use of a CYP3A4 substrate or CYP2C8 substrate with Finerenone had no clinically significant effect on the pharmacokinetics of either the CYP3A4 substrate or CYP2C8 substrate.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a vitro chromosomal aberration assay in cultured Chinese hamster V79 cells, a vivo micronucleus assay done on mice, or a vitro bacterial reverse mutation assay, Finerenone is non-genotoxic.
- Increase in tumor response of Wistar rats or in CD1 mice when given Finerenone was not clinically significant in carcinogenicity studies.
- Fertility was not impaired in male rats receiving Finerenone.
- Fertility was impaired in female rats when given dosages that were 20 times AUC to the maximum human exposure.
Clinical Studies
FIDELIO-DKD Study
- A randomized, double-blind, placebo-controlled, multicenter study that looked into the effects of Finerenone on eGFR.
- The study included 2841 patients who received a placebo compared to the 2833 patients that received Finerenone.
- The patient population had a mean age of 66 years, mostly males (70%), and was mostly White (63%).
- 44 mL/min/1.73m2 is the mean eGFR at baseline of the study.
- 852 mg/g is the median urine albumin-to-creatinine ratio at baseline of the study.
Table 4 summarizes the Primary and Secondary Time-to-Event Endpoints.
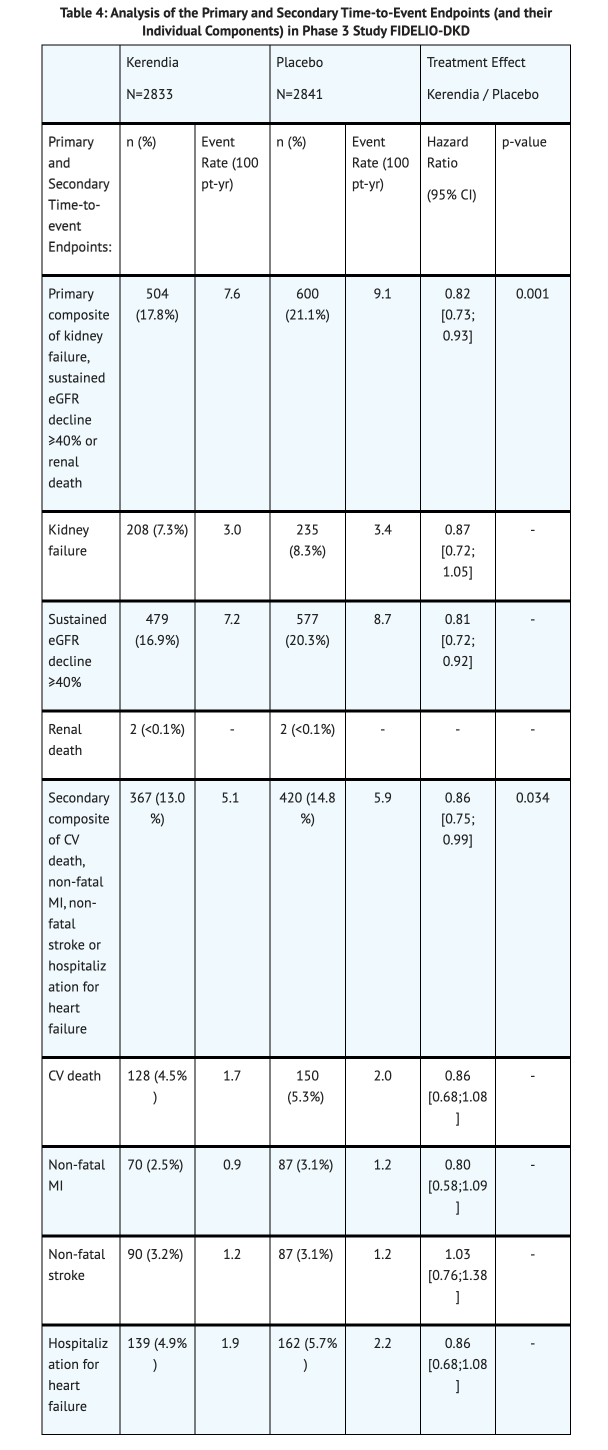
Figure 1 shows the Time to first occurrence of kidney failure, sustained decline in eGFR ≥40% from baseline, or renal death in the FIDELIO-DKD study.
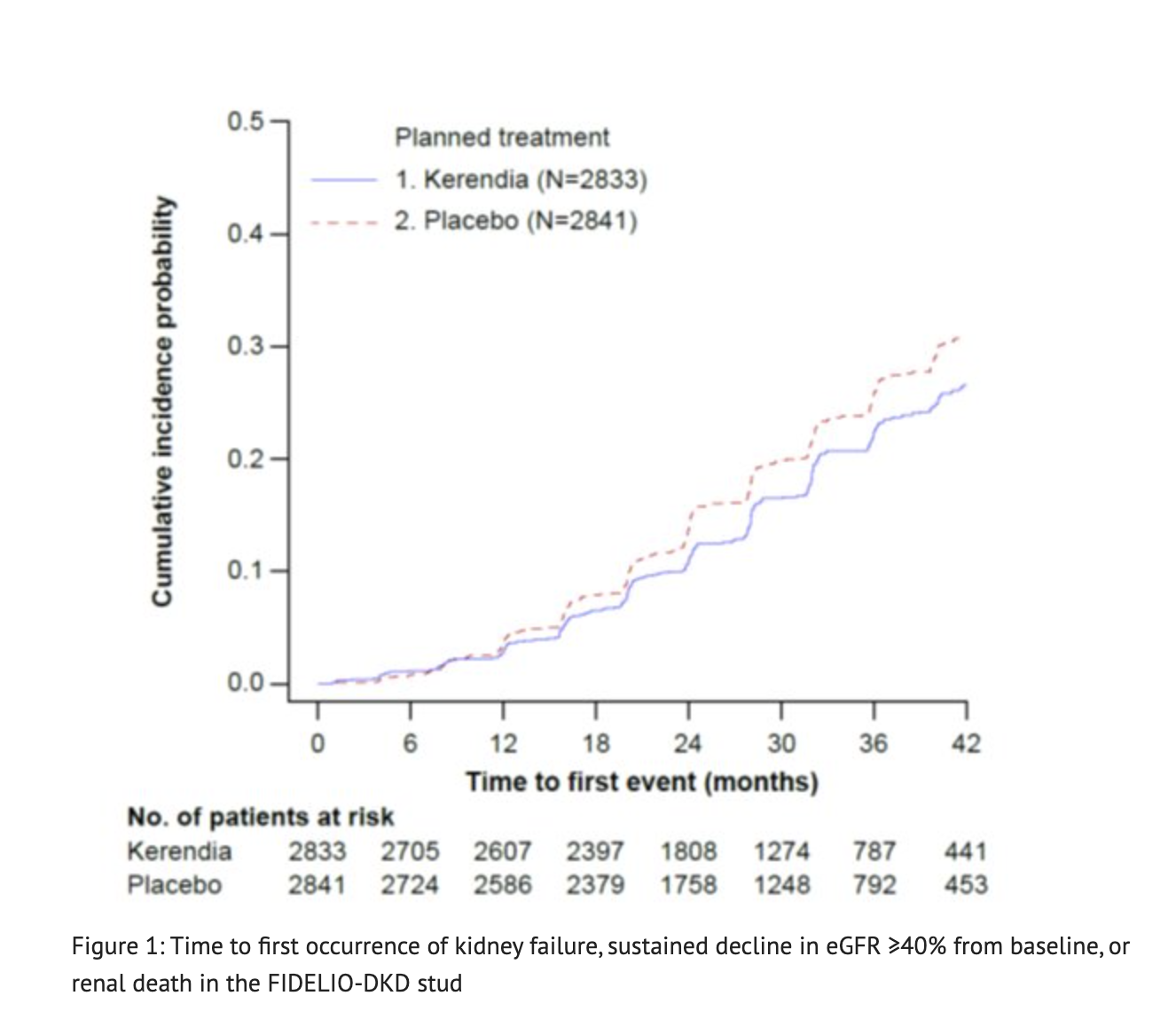
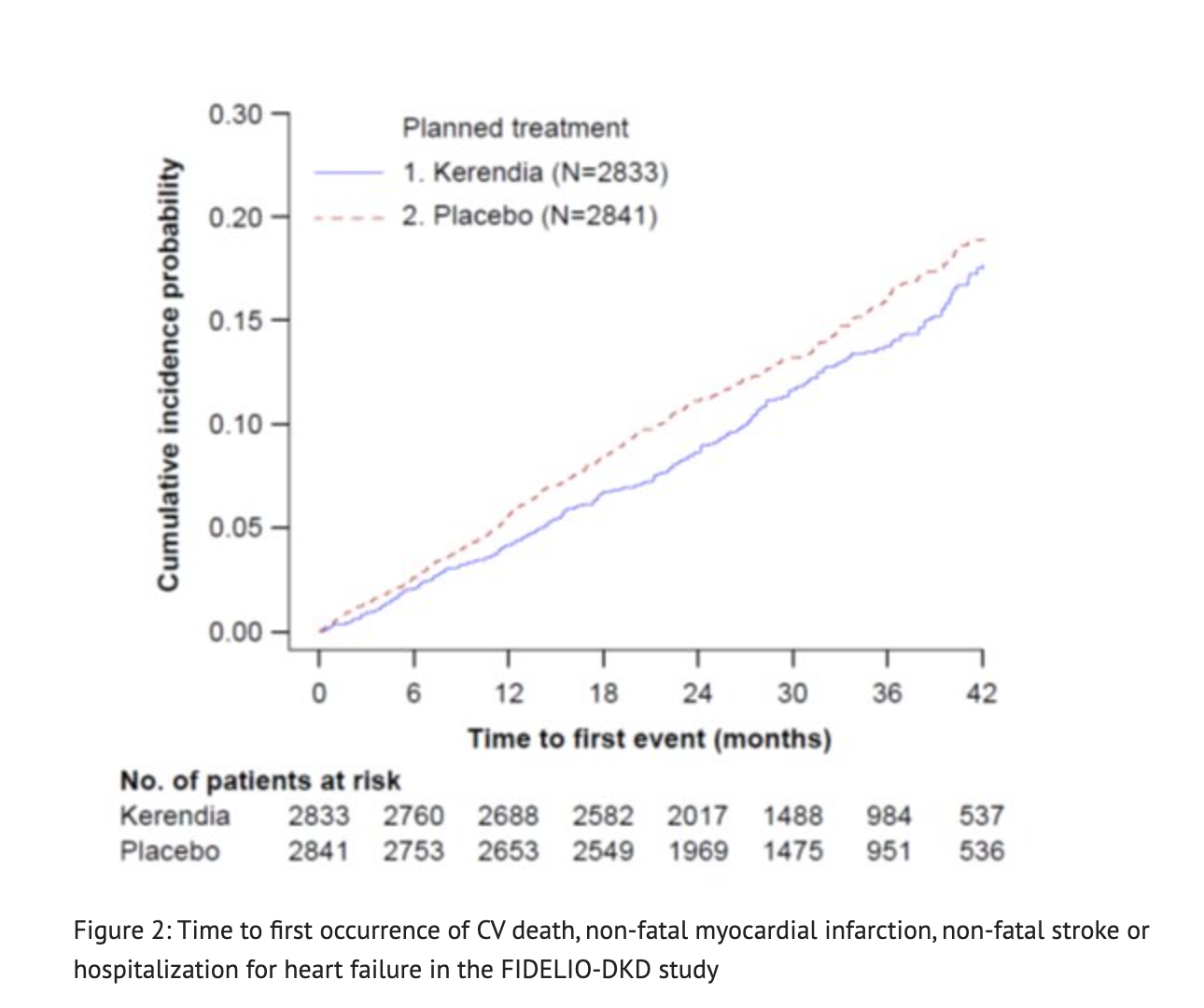
Figure 2 shows the Time to first occurrence of CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for heart failure in the FIDELIO-DKD study.
How Supplied
- Finerenone is either supplied as 10 mg or 20 mg.
- 10 mg of Finerenone tablets are pink oblong.
- 20 mg of Finerenone tablets are yellow oblong.
- Either dosage of Finerenone can be given in 30 or 90 tablet bottles.
Storage
- Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Finerenone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Finerenone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Monitor patients serum potassium levels at baseline and during Finerenone treatment.
- Advise patients using potassium supplements or salt substitutes containing potassium to consult with their medical provider.
- Advise patients to avoid strong or moderate CYP3A4 inducers.
- Advise patients to avoid medications that contain no or weak potential to induce CYP3A4.
- Advise patients to avoid grapefruit or grapefruit juice during Finerenone treatment.
- Advise female patients to avoid nursing during and 1 day after Finerenone treatment.
Precautions with Alcohol
Alcohol-Finerenone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Kerendia
Look-Alike Drug Names
There is limited information regarding Finerenone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.