Ertapenem adverse reactions: Difference between revisions
No edit summary |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 4: | Line 4: | ||
==Adverse Reactions== | ==Adverse Reactions== | ||
===Clinical Trials Experience=== | |||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
Adults Receiving INVANZ as a Treatment Regimen | ====Adults Receiving INVANZ as a Treatment Regimen==== | ||
Clinical trials enrolled 1954 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [see [http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33f3b99b-fa82-42e0-26bf-f49891ae3d22#i4i_clinical_studies_id_4c57f901-8336-444e-9bc8-6629b9090087 Clinical Studies (14)]]. Most adverse experiences reported in these clinical trials were described as mild to moderate in severity. INVANZ was discontinued due to adverse experiences in 4.7% of patients. [http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33f3b99b-fa82-42e0-26bf-f49891ae3d22#TABLE3 Table 3] shows the incidence of adverse experiences reported in ≥2.0% of patients in these trials. The most common drug-related adverse experiences in patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (5.5%), infused vein complication (3.7%), nausea (3.1%), headache (2.2%), and vaginitis in females (2.1%). | Clinical trials enrolled 1954 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [see [http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33f3b99b-fa82-42e0-26bf-f49891ae3d22#i4i_clinical_studies_id_4c57f901-8336-444e-9bc8-6629b9090087 Clinical Studies (14)]]. Most adverse experiences reported in these clinical trials were described as mild to moderate in severity. INVANZ was discontinued due to adverse experiences in 4.7% of patients. [http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=33f3b99b-fa82-42e0-26bf-f49891ae3d22#TABLE3 Table 3] shows the incidence of adverse experiences reported in ≥2.0% of patients in these trials. The most common drug-related adverse experiences in patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (5.5%), infused vein complication (3.7%), nausea (3.1%), headache (2.2%), and vaginitis in females (2.1%). | ||
| Line 23: | Line 22: | ||
In clinical trials, seizure was reported during study therapy plus 14-day follow-up period in 0.5% of patients treated with INVANZ, 0.3% of patients treated with piperacillin/tazobactam and 0% of patients treated with ceftriaxone . | In clinical trials, seizure was reported during study therapy plus 14-day follow-up period in 0.5% of patients treated with INVANZ, 0.3% of patients treated with piperacillin/tazobactam and 0% of patients treated with ceftriaxone . | ||
Additional adverse experiences that were reported with INVANZ with an incidence >0.1% within each body system are listed below | '''''Additional adverse experiences that were reported with INVANZ with an incidence >0.1% within each body system are listed below:''''' | ||
======Body as a Whole====== | |||
Abdominal distention, pain, chills, septicemia, septic shock, dehydration, gout, malaise, asthenia/fatigue, necrosis, candidiasis, weight loss, facial edema, injection site induration, injection site pain, extravasation, phlebitis/thrombophlebitis, flank pain, syncope | |||
======Cardiovascular System====== | |||
Heart failure, hematoma, chest pain, hypertension, tachycardia, cardiac arrest, bradycardia, arrhythmia, atrial fibrillation, heart murmur, ventricular tachycardia, asystole, subdural hemorrhage | |||
======Digestive System====== | |||
Acid regurgitation, oral candidiasis, dyspepsia, gastrointestinal hemorrhage, anorexia, flatulence, C. difficile-associated diarrhea, stomatitis, dysphagia, hemorrhoids, ileus, cholelithiasis, duodenitis, esophagitis, gastritis, jaundice, mouth ulcer, pancreatitis, pyloric stenosis | |||
======Musculoskeletal System====== | |||
Leg pain | |||
Nervous System & Psychiatric | ======Nervous System & Psychiatric====== | ||
Anxiety, nervousness, seizure, tremor, depression, hypesthesia, spasm, paresthesia, aggressive behavior, vertigo | |||
Respiratory System | ======Respiratory System====== | ||
Cough, pharyngitis, rales/rhonchi, respiratory distress, pleural effusion, hypoxemia, bronchoconstriction, pharyngeal discomfort, epistaxis, pleuritic pain, asthma, hemoptysis, hiccups, voice disturbance | |||
Skin & Skin Appendage | ======Skin & Skin Appendage====== | ||
Erythema, sweating, dermatitis, desquamation, flushing, urticaria | |||
Special Senses | ======Special Senses====== | ||
Taste perversion | |||
Urogenital System | ======Urogenital System====== | ||
Renal impairment, oliguria/anuria, vaginal pruritus, hematuria, urinary retention, bladder dysfunction, vaginal candidiasis, vulvovaginitis. | |||
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the adverse experience profile was generally similar to that seen in previous clinical trials. | In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the adverse experience profile was generally similar to that seen in previous clinical trials. | ||
| Line 48: | Line 58: | ||
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post surgery, the overall adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials. Table 4 shows the incidence of adverse experiences other than those previously described above for INVANZ that were reported regardless of causality in ≥2.0% of patients in this trial. | In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post surgery, the overall adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials. Table 4 shows the incidence of adverse experiences other than those previously described above for INVANZ that were reported regardless of causality in ≥2.0% of patients in this trial. | ||
{| | {| | ||
| Line 56: | Line 65: | ||
|} | |} | ||
Additional adverse experiences that were reported in this prophylaxis trial with INVANZ, regardless of causality, with an incidence >0.5% within each body system are listed below: | '''''Additional adverse experiences that were reported in this prophylaxis trial with INVANZ, regardless of causality, with an incidence >0.5% within each body system are listed below:''''' | ||
Gastrointestinal Disorders | ======Gastrointestinal Disorders====== | ||
C. difficile infection or colitis, dry mouth, hematochezia | |||
======General Disorders and Administration Site Condition====== | |||
Crepitations | |||
======Infections and Infestations====== | |||
Cellulitis, abdominal abscess, fungal rash, pelvic abscess | |||
======Injury, Poisoning and Procedural Complications====== | |||
Incision site complication, incision site hemorrhage, intestinal stoma complication, anastomotic leak, seroma, wound dehiscence, wound secretion | |||
======Musculoskeletal and Connective Tissue Disorders====== | |||
Muscle spasms | |||
======Nervous System Disorders====== | |||
Cerebrovascular accident | |||
======Renal and Urinary Disorders====== | |||
Pediatric Patients Receiving INVANZ as a Treatment Regimen | Dysuria, pollakiuria | ||
======Respiratory, Thoracic and Mediastinal Disorders====== | |||
Crackles lung, lung infiltration, pulmonary congestion, pulmonary embolism, wheezing. | |||
====Pediatric Patients Receiving INVANZ as a Treatment Regimen==== | |||
Clinical trials enrolled 384 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [seeClinical Studies (14)]. The overall adverse experience profile in pediatric patients is comparable to that in adult patients. Table 5 shows the incidence of adverse experiences reported in ≥2.0% of pediatric patients in clinical trials. The most common drug-related adverse experiences in pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (6.5%), infusion site pain (5.5%), infusion site erythema (2.6%), vomiting (2.1%). | Clinical trials enrolled 384 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [seeClinical Studies (14)]. The overall adverse experience profile in pediatric patients is comparable to that in adult patients. Table 5 shows the incidence of adverse experiences reported in ≥2.0% of pediatric patients in clinical trials. The most common drug-related adverse experiences in pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (6.5%), infusion site pain (5.5%), infusion site erythema (2.6%), vomiting (2.1%). | ||
{| | {| | ||
|- | |- | ||
| Line 83: | Line 103: | ||
|} | |} | ||
Additional adverse experiences that were reported with INVANZ with an incidence >0.5% within each body system are listed below: | '''''Additional adverse experiences that were reported with INVANZ with an incidence >0.5% within each body system are listed below:''''' | ||
Gastrointestinal Disorders | ======Gastrointestinal Disorders====== | ||
Nausea | |||
General Disorders and Administration Site Condition | ======General Disorders and Administration Site Condition====== | ||
Hypothermia, chest pain, upper abdominal pain; infusion site pruritus, induration, phlebitis, swelling, and warmth | |||
Infections and Infestations | ======Infections and Infestations====== | ||
Candidiasis, oral candidiasis, viral pharyngitis, herpes simplex, ear infection, abdominal abscess | |||
Metabolism and Nutrition Disorders | ======Metabolism and Nutrition Disorders====== | ||
Decreased appetite | |||
M======usculoskeletal and Connective Tissue Disorders====== | |||
Arthralgia | |||
Nervous System Disorders | ======Nervous System Disorders====== | ||
Dizziness, somnolence | |||
Psychiatric Disorders | ======Psychiatric Disorders====== | ||
Insomnia | |||
Reproductive System and Breast Disorders | ======Reproductive System and Breast Disorders====== | ||
Genital rash | |||
Respiratory, Thoracic and Mediastinal Disorders | ======Respiratory, Thoracic and Mediastinal Disorders====== | ||
Wheezing, nasopharyngitis, pleural effusion, rhinitis, rhinorrhea | |||
Skin and Subcutaneous Tissue Disorders | ======Skin and Subcutaneous Tissue Disorders====== | ||
Dermatitis, pruritus, rash erythematous, skin lesion | |||
Vascular Disorders | ======Vascular Disorders====== | ||
Phlebitis. | |||
===Post-Marketing Experience=== | |||
The following additional adverse reactions have been identified during the post-approval use of INVANZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | The following additional adverse reactions have been identified during the post-approval use of INVANZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | ||
Immune System Disorders | ======Immune System Disorders====== | ||
Anaphylaxis including anaphylactoid reactions | |||
Musculoskeletal and Connective Tissue Disorders | ======Musculoskeletal and Connective Tissue Disorders====== | ||
Muscular weakness | |||
Nervous System Disorders | ======Nervous System Disorders====== | ||
Coordination abnormal, depressed level of consciousness, dyskinesia, gait disturbance, myoclonus, tremor | |||
Psychiatric Disorders | ======Psychiatric Disorders====== | ||
Altered mental status (including aggression, delirium), hallucinations | |||
Skin and Subcutaneous Tissue Disorders | ======Skin and Subcutaneous Tissue Disorders====== | ||
Drug Rash with Eosinophilia and Systemic Symptoms (DRESS syndrome) | |||
===Adverse Laboratory Changes in Clinical Trials=== | |||
Adults Receiving INVANZ as Treatment Regimen | ====Adults Receiving INVANZ as Treatment Regimen==== | ||
Laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ in clinical trials are presented in Table 6. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were ALT increased (6.0%), AST increased (5.2%), serum alkaline phosphatase increased (3.4%), and platelet count increased (2.8%). INVANZ was discontinued due to laboratory adverse experiences in 0.3% of patients. | Laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ in clinical trials are presented in Table 6. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were ALT increased (6.0%), AST increased (5.2%), serum alkaline phosphatase increased (3.4%), and platelet count increased (2.8%). INVANZ was discontinued due to laboratory adverse experiences in 0.3% of patients. | ||
| Line 141: | Line 177: | ||
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post surgery, the overall laboratory adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials. | In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post surgery, the overall laboratory adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials. | ||
Pediatric Patients Receiving INVANZ as a Treatment Regimen | ====Pediatric Patients Receiving INVANZ as a Treatment Regimen==== | ||
Laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ in clinical trials are presented in Table 7. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were neutrophil count decreased (3.0%), ALT increased (2.2%), and AST increased (2.1%). | Laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ in clinical trials are presented in Table 7. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were neutrophil count decreased (3.0%), ALT increased (2.2%), and AST increased (2.1%). | ||
Revision as of 00:36, 26 December 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults Receiving INVANZ as a Treatment Regimen
Clinical trials enrolled 1954 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [see Clinical Studies (14)]. Most adverse experiences reported in these clinical trials were described as mild to moderate in severity. INVANZ was discontinued due to adverse experiences in 4.7% of patients. Table 3 shows the incidence of adverse experiences reported in ≥2.0% of patients in these trials. The most common drug-related adverse experiences in patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (5.5%), infused vein complication (3.7%), nausea (3.1%), headache (2.2%), and vaginitis in females (2.1%).
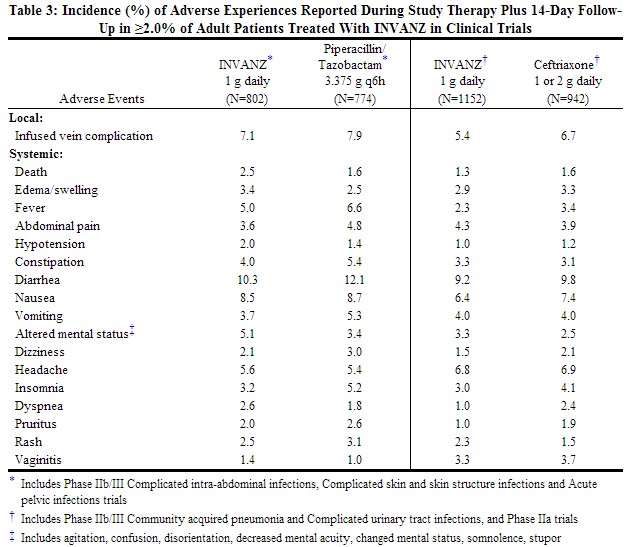 |
In patients treated for complicated intra-abdominal infections, death occurred in 4.7% (15/316) of patients receiving INVANZ and 2.6% (8/307) of patients receiving comparator drug. These deaths occurred in patients with significant co-morbidity and/or severe baseline infections. Deaths were considered unrelated to study drugs by investigators.
In clinical trials, seizure was reported during study therapy plus 14-day follow-up period in 0.5% of patients treated with INVANZ, 0.3% of patients treated with piperacillin/tazobactam and 0% of patients treated with ceftriaxone .
Additional adverse experiences that were reported with INVANZ with an incidence >0.1% within each body system are listed below:
Body as a Whole
Abdominal distention, pain, chills, septicemia, septic shock, dehydration, gout, malaise, asthenia/fatigue, necrosis, candidiasis, weight loss, facial edema, injection site induration, injection site pain, extravasation, phlebitis/thrombophlebitis, flank pain, syncope
Cardiovascular System
Heart failure, hematoma, chest pain, hypertension, tachycardia, cardiac arrest, bradycardia, arrhythmia, atrial fibrillation, heart murmur, ventricular tachycardia, asystole, subdural hemorrhage
Digestive System
Acid regurgitation, oral candidiasis, dyspepsia, gastrointestinal hemorrhage, anorexia, flatulence, C. difficile-associated diarrhea, stomatitis, dysphagia, hemorrhoids, ileus, cholelithiasis, duodenitis, esophagitis, gastritis, jaundice, mouth ulcer, pancreatitis, pyloric stenosis
Musculoskeletal System
Leg pain
Nervous System & Psychiatric
Anxiety, nervousness, seizure, tremor, depression, hypesthesia, spasm, paresthesia, aggressive behavior, vertigo
Respiratory System
Cough, pharyngitis, rales/rhonchi, respiratory distress, pleural effusion, hypoxemia, bronchoconstriction, pharyngeal discomfort, epistaxis, pleuritic pain, asthma, hemoptysis, hiccups, voice disturbance
Skin & Skin Appendage
Erythema, sweating, dermatitis, desquamation, flushing, urticaria
Special Senses
Taste perversion
Urogenital System
Renal impairment, oliguria/anuria, vaginal pruritus, hematuria, urinary retention, bladder dysfunction, vaginal candidiasis, vulvovaginitis.
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the adverse experience profile was generally similar to that seen in previous clinical trials.
Prophylaxis of Surgical Site Infection following Elective Colorectal Surgery
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post surgery, the overall adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials. Table 4 shows the incidence of adverse experiences other than those previously described above for INVANZ that were reported regardless of causality in ≥2.0% of patients in this trial.
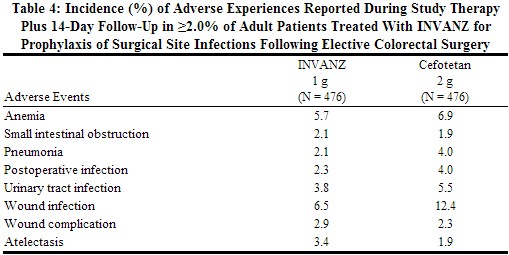 |
Additional adverse experiences that were reported in this prophylaxis trial with INVANZ, regardless of causality, with an incidence >0.5% within each body system are listed below:
Gastrointestinal Disorders
C. difficile infection or colitis, dry mouth, hematochezia
General Disorders and Administration Site Condition
Crepitations
Infections and Infestations
Cellulitis, abdominal abscess, fungal rash, pelvic abscess
Injury, Poisoning and Procedural Complications
Incision site complication, incision site hemorrhage, intestinal stoma complication, anastomotic leak, seroma, wound dehiscence, wound secretion
Musculoskeletal and Connective Tissue Disorders
Muscle spasms
Nervous System Disorders
Cerebrovascular accident
Renal and Urinary Disorders
Dysuria, pollakiuria
Respiratory, Thoracic and Mediastinal Disorders
Crackles lung, lung infiltration, pulmonary congestion, pulmonary embolism, wheezing.
Pediatric Patients Receiving INVANZ as a Treatment Regimen
Clinical trials enrolled 384 patients treated with INVANZ; in some of the clinical trials, parenteral therapy was followed by a switch to an appropriate oral antimicrobial [seeClinical Studies (14)]. The overall adverse experience profile in pediatric patients is comparable to that in adult patients. Table 5 shows the incidence of adverse experiences reported in ≥2.0% of pediatric patients in clinical trials. The most common drug-related adverse experiences in pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, were diarrhea (6.5%), infusion site pain (5.5%), infusion site erythema (2.6%), vomiting (2.1%).
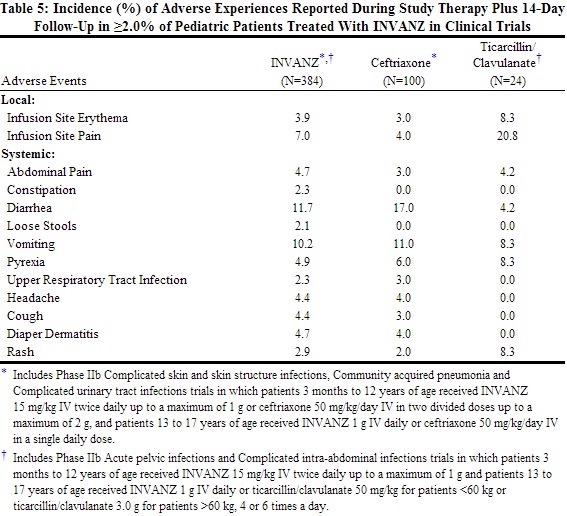 |
Additional adverse experiences that were reported with INVANZ with an incidence >0.5% within each body system are listed below:
Gastrointestinal Disorders
Nausea
General Disorders and Administration Site Condition
Hypothermia, chest pain, upper abdominal pain; infusion site pruritus, induration, phlebitis, swelling, and warmth
Infections and Infestations
Candidiasis, oral candidiasis, viral pharyngitis, herpes simplex, ear infection, abdominal abscess
Metabolism and Nutrition Disorders
Decreased appetite
M======usculoskeletal and Connective Tissue Disorders====== Arthralgia
Nervous System Disorders
Dizziness, somnolence
Psychiatric Disorders
Insomnia
Reproductive System and Breast Disorders
Genital rash
Respiratory, Thoracic and Mediastinal Disorders
Wheezing, nasopharyngitis, pleural effusion, rhinitis, rhinorrhea
Skin and Subcutaneous Tissue Disorders
Dermatitis, pruritus, rash erythematous, skin lesion
Vascular Disorders
Phlebitis.
Post-Marketing Experience
The following additional adverse reactions have been identified during the post-approval use of INVANZ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders
Anaphylaxis including anaphylactoid reactions
Musculoskeletal and Connective Tissue Disorders
Muscular weakness
Nervous System Disorders
Coordination abnormal, depressed level of consciousness, dyskinesia, gait disturbance, myoclonus, tremor
Psychiatric Disorders
Altered mental status (including aggression, delirium), hallucinations
Skin and Subcutaneous Tissue Disorders
Drug Rash with Eosinophilia and Systemic Symptoms (DRESS syndrome)
Adverse Laboratory Changes in Clinical Trials
Adults Receiving INVANZ as Treatment Regimen
Laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ in clinical trials are presented in Table 6. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of adult patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were ALT increased (6.0%), AST increased (5.2%), serum alkaline phosphatase increased (3.4%), and platelet count increased (2.8%). INVANZ was discontinued due to laboratory adverse experiences in 0.3% of patients.
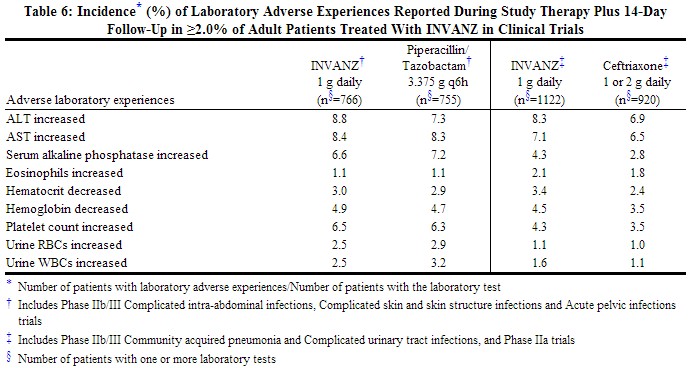 |
Additional laboratory adverse experiences that were reported during therapy in >0.1% of patients treated with INVANZ in clinical trials include: increases in serum creatinine, serum glucose, BUN, total, direct and indirect serum bilirubin, serum sodium and potassium, PT and PTT; decreases in serum potassium, serum albumin, WBC, platelet count, and segmented neutrophils.
In a clinical trial for the treatment of diabetic foot infections in which 289 adult diabetic patients were treated with INVANZ, the laboratory adverse experience profile was generally similar to that seen in previous clinical trials.
Prophylaxis of Surgical Site Infection following Elective Colorectal Surgery
In a clinical trial in adults for the prophylaxis of surgical site infection following elective colorectal surgery in which 476 patients received a 1 g dose of INVANZ 1 hour prior to surgery and were then followed for safety 14 days post surgery, the overall laboratory adverse experience profile was generally comparable to that observed for INVANZ in previous clinical trials.
Pediatric Patients Receiving INVANZ as a Treatment Regimen
Laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ in clinical trials are presented in Table 7. Drug-related laboratory adverse experiences that were reported during therapy in ≥2.0% of pediatric patients treated with INVANZ, including those who were switched to therapy with an oral antimicrobial, in clinical trials were neutrophil count decreased (3.0%), ALT increased (2.2%), and AST increased (2.1%).
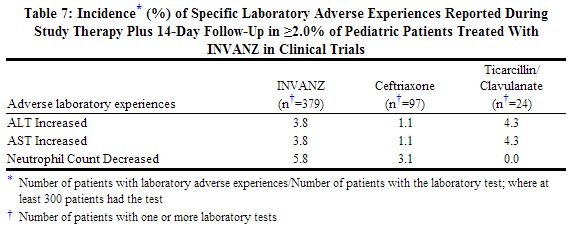 |
Additional laboratory adverse experiences that were reported during therapy in >0.5% of patients treated with INVANZ in clinical trials include: alkaline phosphatase increased, eosinophil count increased, platelet count increased, white blood cell count decreased and urine protein present.[1]
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021337s018lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.