Baeyer-Drewson indigo synthesis: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
(No difference)
|
Revision as of 11:54, 15 October 2010
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
The Baeyer-Drewsen indigo synthesis (1882) is an organic reaction in which indigo is prepared from o-nitrobenzaldehyde and acetone [1] [2]
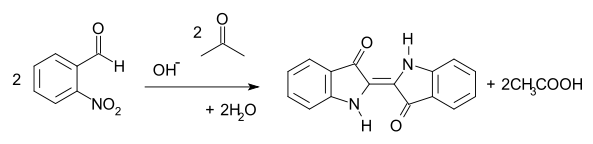
The reaction is classified as a Aldol condensation.
References
- ↑ Adolf Baeyer, Viggo Drewsen (1882). "Darstellung von Indigblau aus Orthonitrobenzaldehyd". Berichte der deutschen chemischen Gesellschaft. 15 (2): 2856–2864. doi:10.1002/cber.188201502274.
- ↑ Helmut Schmidt (1997). "Indigo - 100 Jahre industrielle Synthese". Chemie in unserer Zeit. 31 (3): 121–128. doi:10.1002/ciuz.19970310304.
External links