Sandbox:ab: Difference between revisions
| (208 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
<div style="width: 1px; height: 1px; background-color: #999999; position: fixed; top: 10px; left: 10px"></div> | |||
<div style="width: 90%; -webkit-user-select: none;"> | |||
{| class="infobox" style="margin: 0 0 0 0; border: 0; float: right; width: 5%; background: #A8A8A8; position: fixed; top: 250px; right: 20px; border-radius: 10px 10px 10px 10px;" cellpadding="0" cellspacing="0" ; | |||
|- | |||
! style="padding: 0 5px; font-size: 80%; background: #A8A8A8;" align="center" |{{fontcolor|#2B3B44|Acute leukemia<BR>Resident Survival Guide}} | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#Overview|Overview]] | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#Causes|Causes]] | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#FIRE: Focused Initial Rapid Evaluation|FIRE]] | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#Complete Diagnostic Approach|Diagnosis]] | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#Treatment|Treatment]] | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#Do's|Do's]] | |||
|- | |||
! style="font-size: 80%; padding: 0 5px; background: #DCDCDC; border-radius: 5px 5px 5px 5px;" align="left" |[[{{PAGENAME}}#Don'ts|Don'ts]] | |||
|} | |||
__NOTOC__ | __NOTOC__ | ||
{{ | |||
{{ | {{CMG}}; {{AE}} {{ABehjat}} | ||
{{SK}} Acute lymphocytic leukemia, Acute myeloid leukemia, ALL, AML | |||
==Overview== | ==Overview== | ||
Acute Leukemia is a malignancy of bone marrow myeloid and lymphoblastic precursor cells, in which these poorly differentiated hematopoietic cells proliferate rapidly. Hence, their accumulation would disrupt the performance of bone marrow to produce normal blood cells | |||
<div align="center"><gallery heights="200" widths="200"> | |||
Image:AML_(2).png | |||
Image:ALL2.png | |||
</gallery> | |||
</div> | |||
==Causes== | |||
AML and ALL are life-threatening diseases, which would result in death if left untreated. In the majority of cases, etiology is not apparent. | |||
== | ===Common Causes of AML=== | ||
== | *[[Gene mutations:FLT3, IDHI, IDH2, KRAS, DNMT3A, NPM1]] | ||
*[[Chromosomal translocations, deletions, and inversions]] | |||
*[[ Benzene or radiation exposure chronically]]<ref name="pmid23634996">{{cite journal| author=Cancer Genome Atlas Research Network. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ | display-authors=etal| title=Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. | journal=N Engl J Med | year= 2013 | volume= 368 | issue= 22 | pages= 2059-74 | pmid=23634996 | doi=10.1056/NEJMoa1301689 | pmc=3767041 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23634996 }} </ref> <ref name="pmid8361504">{{cite journal| author=Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H | display-authors=etal| title=Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. | journal=N Engl J Med | year= 1993 | volume= 329 | issue= 13 | pages= 909-14 | pmid=8361504 | doi=10.1056/NEJM199309233291302 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8361504 }} </ref> | |||
===Common Causes of ALL=== | |||
*[[Radiation exposure]] | |||
*[[Genetic disorders; e.g., Down syndrome, ataxia-telangiectasia, Fanconi anemia]] | |||
*[[Certain infections: e.g., HTLV-1]] <ref name="pmid23523389">{{cite journal| author=Inaba H, Greaves M, Mullighan CG| title=Acute lymphoblastic leukaemia. | journal=Lancet | year= 2013 | volume= 381 | issue= 9881 | pages= 1943-55 | pmid=23523389 | doi=10.1016/S0140-6736(12)62187-4 | pmc=3816716 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23523389 }} </ref> | |||
== | ==FIRE== | ||
A Focused Initial Rapid Evaluation (FIRE) should be performed to identify patients in need of immediate intervention. | |||
*Focused Initial Rapid Evaluation (FIRE) in AML <ref name="TallmanWang2019">{{cite journal|last1=Tallman|first1=Martin S.|last2=Wang|first2=Eunice S.|last3=Altman|first3=Jessica K.|last4=Appelbaum|first4=Frederick R.|last5=Bhatt|first5=Vijaya Raj|last6=Bixby|first6=Dale|last7=Coutre|first7=Steven E.|last8=De Lima|first8=Marcos|last9=Fathi|first9=Amir T.|last10=Fiorella|first10=Melanie|last11=Foran|first11=James M.|last12=Hall|first12=Aric C.|last13=Jacoby|first13=Meagan|last14=Lancet|first14=Jeffrey|last15=LeBlanc|first15=Thomas W.|last16=Mannis|first16=Gabriel|last17=Marcucci|first17=Guido|last18=Martin|first18=Michael G.|last19=Mims|first19=Alice|last20=O’Donnell|first20=Margaret R.|last21=Olin|first21=Rebecca|last22=Peker|first22=Deniz|last23=Perl|first23=Alexander|last24=Pollyea|first24=Daniel A.|last25=Pratz|first25=Keith|last26=Prebet|first26=Thomas|last27=Ravandi|first27=Farhad|last28=Shami|first28=Paul J.|last29=Stone|first29=Richard M.|last30=Strickland|first30=Stephen A.|last31=Wieduwilt|first31=Matthew|last32=Gregory|first32=Kristina M.|last33=Hammond|first33=Lydia|last34=Ogba|first34=Ndiya|title=Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology|journal=Journal of the National Comprehensive Cancer Network|volume=17|issue=6|year=2019|pages=721–749|issn=1540-1405|doi=10.6004/jnccn.2019.0028}}</ref><ref>{{cite book | last = Jameson | first = J | title = Harrison's principles of internal medicine | publisher = McGraw-Hill Education | location = New York | year = 2018 | isbn = 978-1259643996 }}</ref>: | |||
* | |||
== | {{Family tree/start}} | ||
{{Family tree | | | | | | | | | | | | | | A01 | | | | | | | | | | | | | | |A01=<div style="float: left; text-align: left; line-height: 150% ">'''Obtain patient's medical history and focus on these signs and symptoms:''' <br> ❑ [[Fatigue]] <br> ❑ [[Weight loss]] <br> ❑ [[Anorexia]] <br> ❑ [[Bone pain]] <br> ❑ [[Bleeding]] <br> ❑ [[Early satiety]] <br> ❑ History of specific and chronic exposures such as alkylating agents, benzene, radiation, or previous chemotherapy <br> ❑ [[Headache]] <br> ❑ [[History of recurrent fever]]</div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | F01 | | | | | | | | | | | | | | |F01=<div style="float: left; text-align: left; line-height: 150% ">'''Examine the patient:''' <br> ❑ [[Fever]] <br> ❑ [[Ecchymosis]] <br> ❑ [[Lymphadenopathy]] <br>❑ [[Splenomegaly]] <br> ❑ [[Hepatomegaly]]<br> ❑ Mediastinal mass <br>❑ Abnormalities in cranial nerve examination <br> ❑ [[Skin Petechiae]]<br> ❑ Testicular enlargement < <br> </div> }} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | B01 | | | | | | | | | | | | | | |B01=<div style="float: left; text-align: left; line-height: 150% ">'''Hematologic evaluation:'''<br> | |||
❑ [[CBD including platelets and WBCs)]] <br> ❑ [[Uric acid]], [[BUN]], [[Cr]], [[Liver function test]]s, [[bilirubin]],[[Ca]], [[P]], [[Sodium]], [[potassium]], <br> ❑ [[amylase]], and [[lipase]] <br> ❑ [[Lactate dehydrogenase]] <br> ❑ [[PT]], [[PTT]] <br> ❑ [[D-dimer]], [[fibrinogen]] <br> ❑ [[Viral antibodies]], [[(varicella-zoster]], [[CMV]]), [[HSV-1]] ❑ Peripheral blood smear</div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | B02 | | | | | | | | | | | | | | | B02= <div style="float: left; text-align: left; line-height: 150% ">'''Radiologic assessment:''' <br> ❑ '''[[CXR]] (PA and lateral) <br> ❑ [[PET]] or [[CT scan]] (if [[extramedullary disease]] is doubted based on symptoms and physical exam) <br> ❑ [[CT]], or [[MRI]], and other imaging methods to diagnose [[ICH]], [[brain]] or [[spinal cord tumor]]s, and [[leptomeningeal disease]] (if patient presenting notable CNS signs and symptoms <br> </div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | E01 | | | | | | | | | | | | | | |E01=<div style="float: left; text-align: left; line-height: 150% ">''' ❑ [[Bone marrow aspiration]] and biopsy <br> </div>}} | |||
{{Family tree | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |}} | |||
{{familytree/end}} | |||
*Focused Initial Rapid Evaluation (FIRE) in ALL:<ref name="pmid23523389">{{cite journal| author=Inaba H, Greaves M, Mullighan CG| title=Acute lymphoblastic leukaemia. | journal=Lancet | year= 2013 | volume= 381 | issue= 9881 | pages= 1943-55 | pmid=23523389 | doi=10.1016/S0140-6736(12)62187-4 | pmc=3816716 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23523389 }} </ref> <ref name="pmid31910389">{{cite journal| author=Brown P, Inaba H, Annesley C, Beck J, Colace S, Dallas M | display-authors=etal| title=Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. | journal=J Natl Compr Canc Netw | year= 2020 | volume= 18 | issue= 1 | pages= 81-112 | pmid=31910389 | doi=10.6004/jnccn.2020.0001 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=31910389 }} </ref> <ref name="pmid25060251">{{cite journal| author=Rose-Inman H, Kuehl D| title=Acute leukemia. | journal=Emerg Med Clin North Am | year= 2014 | volume= 32 | issue= 3 | pages= 579-96 | pmid=25060251 | doi=10.1016/j.emc.2014.04.004 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25060251 }} </ref> | |||
{{Family tree/start}} | |||
{{Family tree | | | | | | | | | | | | | | A01 | | | | | | | | | | | | | | |A01=<div style="float: left; text-align: left; line-height: 150% ">'''Take a precise medical history and focus on these signs and symptoms:''' <br> ❑ [[Fatigue]] <br> ❑ [[Anorexia]] <br> ❑ [[Bone and joint pain]] <br> ❑ [[Bleeding]] <br> ❑ [[Weakness and lethargy]] <br> ❑ History of prior exposures with alkylating agents, radiation, or previous chemotherapy (less prevalent than AML) <br> ❑ [[Headache]] <br> ❑ [[History of bleeding or unexplained bruising]] <br> ❑ History of congenital syndroms <br> ❑ [[Night sweats, weight loss and fever(B symptoms)]] <br>❑ [[Abdominal distention]] <br></div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | F01 | | | | | | | | | | | | | | |F01=<div style="float: left; text-align: left; line-height: 150% ">'''Examine the patient:''' <br> ❑ [[Fever]] <br> ❑ [[Tachycardia]]<br> ❑ Mediastinal mass <br> ❑ [[Lymphadenopathy]] <br>❑ [[Splenomegaly]] <br> ❑ [[Hepatomegaly]]<br> ❑ Abnormalities in cranial nerve examination <br> ❑ [[Skin Petechiae]] <br> ❑Testicular enlargement (rare) <br> </div> }} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | B01 | | | | | | | | | | | | | | |B01=<div style="float: left; text-align: left; line-height: 150% ">'''Hematologic evaluation:'''<br> | |||
❑ [[CBD including platelets and WBCs)]] <br> ❑ [[Uric acid]], [[BUN]], [[Cr]], [[Liver function test]]s, [[bilirubin]] <br> ❑ [[Lactate dehydrogenase]] , potassium, phosphates, and calcium <br> ❑ d-dimer, fibrinogen, PT, and PTT <br> ❑ Peripheral blood smear <br> </div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | B02 | | | | | | | | | | | | | | | B02= <div style="float: left; text-align: left; line-height: 150% ">'''Radiologic assessment:''' <br> ❑ '''[[CXR]] (PA and lateral) to rule out mediastinal masses <br> ❑ Brain CT scan and MRI with contrast if neurologic signs and symptoms have existed <br> ❑ Scrotal ultrasound for assessing testicular involvement <br> ❑ Echocardiogram or cardiac scan <br> A whole body PET or CT scan when lymphoblastic lymphoma is doubted </div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | E01 | | | | | | | | | | | | | | |E01=<div style="float: left; text-align: left; line-height: 150% ">''' ❑ [[Bone marrow aspiration]] and biopsy <br> </div>}} | |||
{{Family tree | | | | | | | | | | | | | | |!| | | | | | | | | | | | | | | |}} | |||
{{Family tree | | | | | | | | | | | | | | E01 | | | | | | | | | | | | | | |E01=<div style="float: left; text-align: left; line-height: 150% ">''' ❑ [[Lumbar puncture]] <br> </div>}} | |||
{{Family tree | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |}} | |||
{{familytree/end}} | |||
== | ==Diagnosis== | ||
According to | Diagnostic criteria of acute myeloid leukemia and acute lymphoblastic leukemia are similar to one another. | ||
*According to the 2016 WHO criteria observing ≥20% blasts in the bone marrow biopsy or peripheral blood smear is diagnostic for AML. These genetic abnormalities in AML are diagnostic even with less than 20% marrow blasts: inv(16), t(16;16), t(8;21), and t(15;17).<ref name="pmid10643532">{{cite journal| author=Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J | display-authors=etal| title=The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. | journal=Ann Oncol | year= 1999 | volume= 10 | issue= 12 | pages= 1419-32 | pmid=10643532 | doi=10.1023/a:1008375931236 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10643532 }} </ref> | |||
== Diagnosis == | *Presenting ≥20% of leukemic lymphoblasts in bone marrow aspirate and biopsy would prove ALL.<ref name="pmid25408859">{{cite journal| author=Chiaretti S, Zini G, Bassan R| title=Diagnosis and subclassification of acute lymphoblastic leukemia. | journal=Mediterr J Hematol Infect Dis | year= 2014 | volume= 6 | issue= 1 | pages= e2014073 | pmid=25408859 | doi=10.4084/MJHID.2014.073 | pmc=4235437 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25408859 }} </ref> | ||
=== | |||
=== | |||
==Treatment== | |||
: | {{familytree/start |summary=Sample 6}} | ||
: | {{familytree | | | | | | | | | | | | | | | | | | A01 |A01= <div style="float: left; text-align: left; width: 25em; padding:1em;">'''Treatment of a patient with definitive AML'''<ref name="pmid26376137">{{cite journal| author=Döhner H, Weisdorf DJ, Bloomfield CD| title=Acute Myeloid Leukemia. | journal=N Engl J Med | year= 2015 | volume= 373 | issue= 12 | pages= 1136-52 | pmid=26376137 | doi=10.1056/NEJMra1406184 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=26376137 }} </ref> <ref name="pmid27895058">{{cite journal| author=Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T | display-authors=etal| title=Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. | journal=Blood | year= 2017 | volume= 129 | issue= 4 | pages= 424-447 | pmid=27895058 | doi=10.1182/blood-2016-08-733196 | pmc=5291965 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27895058 }} </ref> <ref>{{cite book | last = Jameson | first = J | title = Harrison's principles of internal medicine | publisher = McGraw-Hill Education | location = New York | year = 2018 | isbn = 978-1259643996 }}</ref> | ||
:* | </div>}} | ||
: | {{familytree | | | | | | | | | |,|-|-|-|-|-|-|-|-|^|-|-|-|-|-|-|-|-|-|-|-|-|-|-|-|-|-|-|-|.| | | }} | ||
{{familytree | | | | | | | | | B01 | | | | | | | | | | | | | | | | | | | | | | | | | | | B02 | |B01=<div style="float: left; text-align: left; width: 25em; padding:1em;">'''Treating for the first time(new case)''' </div>|B02= <div style="float: left; text-align: left; width: 25em; padding:1em;">'''Cases with relapsed/refractory AML''' </div>}} | |||
{{familytree | |,|-|-|-|-|-|-|-|+|-|-|-|-|-|-|-|-|-|-|.| | | | | | | | | | | | | | | | | |!| | }} | |||
{{familytree | C01 | | | | | | C02 | | | | | | | | | C03 | | | | | | | | | | | | | | | | C04 |C01= <div style="float: left; text-align: left; width: 25em; padding:1em;">'''Favorable-Risk Cytogenetics''' </div>|C02= <div style="float: left; text-align: left; width: 25em; padding:1em;">'''Intermediate-Risk Cytogenetics''' </div> | |||
|C03= <div style="float: left; text-align: left; width: 25em; padding:1em;">'''Poor-Risk Cytogenetics|C04= <div style="float: left; text-align: left; width: 25em; padding:1em;">'''Salvage therapy''' </div>}} | |||
{{familytree | |!| | | | | | | |!| | | | | | | | | | |!| | | | |!| | | | | | | | | | | | |!| | }} | |||
{{familytree | D01 | | | | | | D02 | | | | | | | | | D03 | | | | | | | | | | | | | | | | D04 | |D01= Select one of these therapies: <br> ❑ 1. Induction treatment: Cytarabine-based regimen + Daunorubicin. If a complete remission is achieved, postremission consolidation therapy as maintenance should be started: intermediate dose of Cytarabine) <br> ❑ 2.Investigational drugs(clinical trial): for cases aged younger than 60 years, a standard chemotherapy regimen including a backbone of Cytarabine + Anthracycline has been recommended. After complete remission, postremission therapy have to be regarded. | |||
|D02=Select one of these treatments: <br> | |||
❑ 1. Investigational therapy: for patients older than 65 or have more comorbidities and high-risk illnesses or cases who cannot tolerate Cytarabine-based regimen + Daunorubicin, changing their regiments to an investigational therapy could be regarded. (administrate one chemotherapy drug or combine their treatment with non-intensive medications fitting with the patient such as decitabine, azacitidine). After complete remission, postremission therapy have to be regarded.<br> ❑ 2. Induction treatment: Cytarabine-based regimen + Daunorubicin (could be suitable for young and elderly patients). If complete remission(CR) is achieved, postremission consolidation therapy as maintenance should be started to prevent relapse, including: <br> | |||
:* Allogenic hematopoietic cell transplantation, which is the preferred treatment, or <br> | |||
:* If the patient is younger than 60 years autologous hematopoietic cell transplantation is recommended, or <br> | |||
:* Intermediate dose of Cytarabine | |||
||D03= Select one of these therapies:<br> 1. Induction treatment: Cytarabine-based regimen + Daunorubicin (could be suitable for both young and elderly patients). After complete remission(CR) achieved, postremission consolidation therapy should be started to prevent relapse, including Allogenic hematopoietic cell transplantation, which is the preferred treatment. When there is not any accessible HLA-matched donor, using an alternate donor has been recommended. <br> 2. Investigational therapy: for patients older than 65 or have more comorbidities and high-risk illnesses or cases who cannot tolerate Cytarabine-based regimen + Daunorubicin, changing their regiments to an investigational therapy could be regarded. (administrate one chemotherapy drug or combine their treatment with non-intensive medications fitting with the patient such as decitabine, azacitidine). After complete remission, postremission therapy has to be regarded.<br> |D04=Patients with relapsed and refractory AML who has an HLA-matched donor accessible for allogenic HCT or cases who attained second complete remission after salvage therapy while there is an available appropriate donor should undergo allogenic hematopoietic cell transplantation. Those who do not have these conditions have to be treated based on clinical trials (investigational therapy). }} | |||
{{familytree/end}} | |||
Treatment of acute lymphoblastic leukemia includes three phases:<ref name="pmid21220592">{{cite journal| author=Bassan R, Hoelzer D| title=Modern therapy of acute lymphoblastic leukemia. | journal=J Clin Oncol | year= 2011 | volume= 29 | issue= 5 | pages= 532-43 | pmid=21220592 | doi=10.1200/JCO.2010.30.1382 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21220592 }} </ref> | |||
: | |||
: | |||
:*Induction therapy, i.e., prednisolone, vincristine, cytarabine <br> | |||
* | :*Administrating Central Nervous System prophylaxis, i.e., methotrexate | ||
:*Chemotherapy as a maintenance treatment for two years | |||
:*Stem cell transplantation in adults who are eligible while there is a suitable donor | |||
==Do's== | |||
:* Before starting the therapy, taking a precise history and physical examination have to be done to diagnose any kind of comorbidities ,i.e. heart failure or renal diseases that affect the prognosis and treatment choices. | |||
== | |||
* | |||
:*HLA-typing evaluation have to be done for all of the AML cases In the pretreatment assessment. | |||
:*Seven days after the induction phase of chemotherapy ended, bone marrow biopsy must be done in order to assess the remission situation. | |||
* | :* In the induction chemotherapy process of AML for most of the cases, cytarabine IV infusion should be administrated for seven days consecutively + anthracycline on days one to three.(known as "7+3" regimens)<ref name="pmidhttps://pubmed.ncbi.nlm.nih.gov/9045305">{{cite journal| author=Bishop JF| title=The treatment of adult acute myeloid leukemia. | journal=Semin Oncol | year= 1997 | volume= 24 | issue= 1 | pages= 57-69 | pmid=https://pubmed.ncbi.nlm.nih.gov/9045305 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9045305 }} </ref> <ref name="TallmanWang2019">{{cite journal|last1=Tallman|first1=Martin S.|last2=Wang|first2=Eunice S.|last3=Altman|first3=Jessica K.|last4=Appelbaum|first4=Frederick R.|last5=Bhatt|first5=Vijaya Raj|last6=Bixby|first6=Dale|last7=Coutre|first7=Steven E.|last8=De Lima|first8=Marcos|last9=Fathi|first9=Amir T.|last10=Fiorella|first10=Melanie|last11=Foran|first11=James M.|last12=Hall|first12=Aric C.|last13=Jacoby|first13=Meagan|last14=Lancet|first14=Jeffrey|last15=LeBlanc|first15=Thomas W.|last16=Mannis|first16=Gabriel|last17=Marcucci|first17=Guido|last18=Martin|first18=Michael G.|last19=Mims|first19=Alice|last20=O’Donnell|first20=Margaret R.|last21=Olin|first21=Rebecca|last22=Peker|first22=Deniz|last23=Perl|first23=Alexander|last24=Pollyea|first24=Daniel A.|last25=Pratz|first25=Keith|last26=Prebet|first26=Thomas|last27=Ravandi|first27=Farhad|last28=Shami|first28=Paul J.|last29=Stone|first29=Richard M.|last30=Strickland|first30=Stephen A.|last31=Wieduwilt|first31=Matthew|last32=Gregory|first32=Kristina M.|last33=Hammond|first33=Lydia|last34=Ogba|first34=Ndiya|title=Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology|journal=Journal of the National Comprehensive Cancer Network|volume=17|issue=6|year=2019|pages=721–749|issn=1540-1405|doi=10.6004/jnccn.2019.0028}}</ref> | ||
* | |||
=== | |||
=== | |||
* | ==Don'ts== | ||
:*If the patient with AML has a coagulopathy disorder and is susceptible to bleeding, do not have to undergo lumbar puncture in the workup process before correcting that. | |||
:*Without the assessment of cardiac symptoms and echocardiogram, chemotherapy medications which are cardiotoxic should not be administrated. <ref name="TallmanWang2019">{{cite journal|last1=Tallman|first1=Martin S.|last2=Wang|first2=Eunice S.|last3=Altman|first3=Jessica K.|last4=Appelbaum|first4=Frederick R.|last5=Bhatt|first5=Vijaya Raj|last6=Bixby|first6=Dale|last7=Coutre|first7=Steven E.|last8=De Lima|first8=Marcos|last9=Fathi|first9=Amir T.|last10=Fiorella|first10=Melanie|last11=Foran|first11=James M.|last12=Hall|first12=Aric C.|last13=Jacoby|first13=Meagan|last14=Lancet|first14=Jeffrey|last15=LeBlanc|first15=Thomas W.|last16=Mannis|first16=Gabriel|last17=Marcucci|first17=Guido|last18=Martin|first18=Michael G.|last19=Mims|first19=Alice|last20=O’Donnell|first20=Margaret R.|last21=Olin|first21=Rebecca|last22=Peker|first22=Deniz|last23=Perl|first23=Alexander|last24=Pollyea|first24=Daniel A.|last25=Pratz|first25=Keith|last26=Prebet|first26=Thomas|last27=Ravandi|first27=Farhad|last28=Shami|first28=Paul J.|last29=Stone|first29=Richard M.|last30=Strickland|first30=Stephen A.|last31=Wieduwilt|first31=Matthew|last32=Gregory|first32=Kristina M.|last33=Hammond|first33=Lydia|last34=Ogba|first34=Ndiya|title=Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology|journal=Journal of the National Comprehensive Cancer Network|volume=17|issue=6|year=2019|pages=721–749|issn=1540-1405|doi=10.6004/jnccn.2019.0028}}</ref> | |||
==References== | ==References== | ||
{{ | {{reflist|2}} | ||
[[Category:Disease]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
[[Category:Resident survival guide]] | |||
Latest revision as of 19:00, 3 November 2020
| Acute leukemia Resident Survival Guide |
|---|
| Overview |
| Causes |
| FIRE |
| Diagnosis |
| Treatment |
| Do's |
| Don'ts |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alieh Behjat, M.D.[2]
Synonyms and keywords: Acute lymphocytic leukemia, Acute myeloid leukemia, ALL, AML
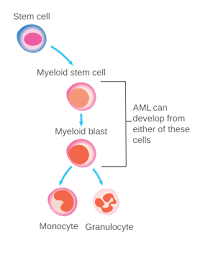
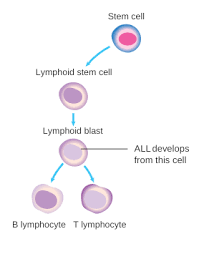
Overview
Acute Leukemia is a malignancy of bone marrow myeloid and lymphoblastic precursor cells, in which these poorly differentiated hematopoietic cells proliferate rapidly. Hence, their accumulation would disrupt the performance of bone marrow to produce normal blood cells
Causes
AML and ALL are life-threatening diseases, which would result in death if left untreated. In the majority of cases, etiology is not apparent.
Common Causes of AML
- Gene mutations:FLT3, IDHI, IDH2, KRAS, DNMT3A, NPM1
- Chromosomal translocations, deletions, and inversions
- Benzene or radiation exposure chronically[1] [2]
Common Causes of ALL
- Radiation exposure
- Genetic disorders; e.g., Down syndrome, ataxia-telangiectasia, Fanconi anemia
- Certain infections: e.g., HTLV-1 [3]
FIRE
A Focused Initial Rapid Evaluation (FIRE) should be performed to identify patients in need of immediate intervention.
Obtain patient's medical history and focus on these signs and symptoms: ❑ Fatigue ❑ Weight loss ❑ Anorexia ❑ Bone pain ❑ Bleeding ❑ Early satiety ❑ History of specific and chronic exposures such as alkylating agents, benzene, radiation, or previous chemotherapy ❑ Headache ❑ History of recurrent fever | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Examine the patient: ❑ Fever ❑ Ecchymosis ❑ Lymphadenopathy ❑ Splenomegaly ❑ Hepatomegaly ❑ Mediastinal mass ❑ Abnormalities in cranial nerve examination ❑ Skin Petechiae ❑ Testicular enlargement < | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hematologic evaluation: ❑ CBD including platelets and WBCs) ❑ Uric acid, BUN, Cr, Liver function tests, bilirubin,Ca, P, Sodium, potassium, ❑ amylase, and lipase ❑ Lactate dehydrogenase ❑ PT, PTT ❑ D-dimer, fibrinogen ❑ Viral antibodies, (varicella-zoster, CMV), HSV-1 ❑ Peripheral blood smear | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Radiologic assessment: ❑ CXR (PA and lateral) ❑ PET or CT scan (if extramedullary disease is doubted based on symptoms and physical exam) ❑ CT, or MRI, and other imaging methods to diagnose ICH, brain or spinal cord tumors, and leptomeningeal disease (if patient presenting notable CNS signs and symptoms | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
❑ Bone marrow aspiration and biopsy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Take a precise medical history and focus on these signs and symptoms: ❑ Fatigue ❑ Anorexia ❑ Bone and joint pain ❑ Bleeding ❑ Weakness and lethargy ❑ History of prior exposures with alkylating agents, radiation, or previous chemotherapy (less prevalent than AML) ❑ Headache ❑ History of bleeding or unexplained bruising ❑ History of congenital syndroms ❑ Night sweats, weight loss and fever(B symptoms) ❑ Abdominal distention | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Examine the patient: ❑ Fever ❑ Tachycardia ❑ Mediastinal mass ❑ Lymphadenopathy ❑ Splenomegaly ❑ Hepatomegaly ❑ Abnormalities in cranial nerve examination ❑ Skin Petechiae ❑Testicular enlargement (rare) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hematologic evaluation: ❑ CBD including platelets and WBCs) ❑ Uric acid, BUN, Cr, Liver function tests, bilirubin ❑ Lactate dehydrogenase , potassium, phosphates, and calcium ❑ d-dimer, fibrinogen, PT, and PTT ❑ Peripheral blood smear | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Radiologic assessment: ❑ CXR (PA and lateral) to rule out mediastinal masses ❑ Brain CT scan and MRI with contrast if neurologic signs and symptoms have existed ❑ Scrotal ultrasound for assessing testicular involvement ❑ Echocardiogram or cardiac scan A whole body PET or CT scan when lymphoblastic lymphoma is doubted | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
❑ Bone marrow aspiration and biopsy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Diagnosis
Diagnostic criteria of acute myeloid leukemia and acute lymphoblastic leukemia are similar to one another.
- According to the 2016 WHO criteria observing ≥20% blasts in the bone marrow biopsy or peripheral blood smear is diagnostic for AML. These genetic abnormalities in AML are diagnostic even with less than 20% marrow blasts: inv(16), t(16;16), t(8;21), and t(15;17).[8]
- Presenting ≥20% of leukemic lymphoblasts in bone marrow aspirate and biopsy would prove ALL.[9]
Treatment
Treating for the first time(new case) | Cases with relapsed/refractory AML | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Favorable-Risk Cytogenetics | Intermediate-Risk Cytogenetics | Poor-Risk Cytogenetics | Salvage therapy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Select one of these therapies: ❑ 1. Induction treatment: Cytarabine-based regimen + Daunorubicin. If a complete remission is achieved, postremission consolidation therapy as maintenance should be started: intermediate dose of Cytarabine) ❑ 2.Investigational drugs(clinical trial): for cases aged younger than 60 years, a standard chemotherapy regimen including a backbone of Cytarabine + Anthracycline has been recommended. After complete remission, postremission therapy have to be regarded. | Select one of these treatments: ❑ 1. Investigational therapy: for patients older than 65 or have more comorbidities and high-risk illnesses or cases who cannot tolerate Cytarabine-based regimen + Daunorubicin, changing their regiments to an investigational therapy could be regarded. (administrate one chemotherapy drug or combine their treatment with non-intensive medications fitting with the patient such as decitabine, azacitidine). After complete remission, postremission therapy have to be regarded.
| Select one of these therapies: 1. Induction treatment: Cytarabine-based regimen + Daunorubicin (could be suitable for both young and elderly patients). After complete remission(CR) achieved, postremission consolidation therapy should be started to prevent relapse, including Allogenic hematopoietic cell transplantation, which is the preferred treatment. When there is not any accessible HLA-matched donor, using an alternate donor has been recommended. 2. Investigational therapy: for patients older than 65 or have more comorbidities and high-risk illnesses or cases who cannot tolerate Cytarabine-based regimen + Daunorubicin, changing their regiments to an investigational therapy could be regarded. (administrate one chemotherapy drug or combine their treatment with non-intensive medications fitting with the patient such as decitabine, azacitidine). After complete remission, postremission therapy has to be regarded. | Patients with relapsed and refractory AML who has an HLA-matched donor accessible for allogenic HCT or cases who attained second complete remission after salvage therapy while there is an available appropriate donor should undergo allogenic hematopoietic cell transplantation. Those who do not have these conditions have to be treated based on clinical trials (investigational therapy). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Treatment of acute lymphoblastic leukemia includes three phases:[13]
- Induction therapy, i.e., prednisolone, vincristine, cytarabine
- Administrating Central Nervous System prophylaxis, i.e., methotrexate
- Chemotherapy as a maintenance treatment for two years
- Stem cell transplantation in adults who are eligible while there is a suitable donor
- Induction therapy, i.e., prednisolone, vincristine, cytarabine
Do's
- Before starting the therapy, taking a precise history and physical examination have to be done to diagnose any kind of comorbidities ,i.e. heart failure or renal diseases that affect the prognosis and treatment choices.
- HLA-typing evaluation have to be done for all of the AML cases In the pretreatment assessment.
- Seven days after the induction phase of chemotherapy ended, bone marrow biopsy must be done in order to assess the remission situation.
- In the induction chemotherapy process of AML for most of the cases, cytarabine IV infusion should be administrated for seven days consecutively + anthracycline on days one to three.(known as "7+3" regimens)[14] [4]
Don'ts
- If the patient with AML has a coagulopathy disorder and is susceptible to bleeding, do not have to undergo lumbar puncture in the workup process before correcting that.
- Without the assessment of cardiac symptoms and echocardiogram, chemotherapy medications which are cardiotoxic should not be administrated. [4]
References
- ↑ Cancer Genome Atlas Research Network. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ; et al. (2013). "Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia". N Engl J Med. 368 (22): 2059–74. doi:10.1056/NEJMoa1301689. PMC 3767041. PMID 23634996.
- ↑ Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, McCabe NR, Kobayashi H; et al. (1993). "Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations". N Engl J Med. 329 (13): 909–14. doi:10.1056/NEJM199309233291302. PMID 8361504.
- ↑ 3.0 3.1 Inaba H, Greaves M, Mullighan CG (2013). "Acute lymphoblastic leukaemia". Lancet. 381 (9881): 1943–55. doi:10.1016/S0140-6736(12)62187-4. PMC 3816716. PMID 23523389.
- ↑ 4.0 4.1 4.2 Tallman, Martin S.; Wang, Eunice S.; Altman, Jessica K.; Appelbaum, Frederick R.; Bhatt, Vijaya Raj; Bixby, Dale; Coutre, Steven E.; De Lima, Marcos; Fathi, Amir T.; Fiorella, Melanie; Foran, James M.; Hall, Aric C.; Jacoby, Meagan; Lancet, Jeffrey; LeBlanc, Thomas W.; Mannis, Gabriel; Marcucci, Guido; Martin, Michael G.; Mims, Alice; O’Donnell, Margaret R.; Olin, Rebecca; Peker, Deniz; Perl, Alexander; Pollyea, Daniel A.; Pratz, Keith; Prebet, Thomas; Ravandi, Farhad; Shami, Paul J.; Stone, Richard M.; Strickland, Stephen A.; Wieduwilt, Matthew; Gregory, Kristina M.; Hammond, Lydia; Ogba, Ndiya (2019). "Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology". Journal of the National Comprehensive Cancer Network. 17 (6): 721–749. doi:10.6004/jnccn.2019.0028. ISSN 1540-1405.
- ↑ Jameson, J (2018). Harrison's principles of internal medicine. New York: McGraw-Hill Education. ISBN 978-1259643996.
- ↑ Brown P, Inaba H, Annesley C, Beck J, Colace S, Dallas M; et al. (2020). "Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology". J Natl Compr Canc Netw. 18 (1): 81–112. doi:10.6004/jnccn.2020.0001. PMID 31910389.
- ↑ Rose-Inman H, Kuehl D (2014). "Acute leukemia". Emerg Med Clin North Am. 32 (3): 579–96. doi:10.1016/j.emc.2014.04.004. PMID 25060251.
- ↑ Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J; et al. (1999). "The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997". Ann Oncol. 10 (12): 1419–32. doi:10.1023/a:1008375931236. PMID 10643532.
- ↑ Chiaretti S, Zini G, Bassan R (2014). "Diagnosis and subclassification of acute lymphoblastic leukemia". Mediterr J Hematol Infect Dis. 6 (1): e2014073. doi:10.4084/MJHID.2014.073. PMC 4235437. PMID 25408859.
- ↑ Döhner H, Weisdorf DJ, Bloomfield CD (2015). "Acute Myeloid Leukemia". N Engl J Med. 373 (12): 1136–52. doi:10.1056/NEJMra1406184. PMID 26376137.
- ↑ Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T; et al. (2017). "Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel". Blood. 129 (4): 424–447. doi:10.1182/blood-2016-08-733196. PMC 5291965. PMID 27895058.
- ↑ Jameson, J (2018). Harrison's principles of internal medicine. New York: McGraw-Hill Education. ISBN 978-1259643996.
- ↑ Bassan R, Hoelzer D (2011). "Modern therapy of acute lymphoblastic leukemia". J Clin Oncol. 29 (5): 532–43. doi:10.1200/JCO.2010.30.1382. PMID 21220592.
- ↑ Bishop JF (1997). "The treatment of adult acute myeloid leukemia". Semin Oncol. 24 (1): 57–69. PMID https://pubmed.ncbi.nlm.nih.gov/9045305 Check
|pmid=value (help).