|
|
| (98 intermediate revisions by 2 users not shown) |
| Line 1: |
Line 1: |
| | ==Physical examination== |
| | ==References== |
| | {{reflist|2}} |
| | |
| | {{WH}} |
| | {{WS}} |
| | |
| | ==References== |
| | {{Reflist|2}} |
| | |
| | |
| ===Pathophysiology prev=== | | ===Pathophysiology prev=== |
| <div style="-webkit-user-select: none;"> | | <div style="-webkit-user-select: none;"> |
| Line 9: |
Line 20: |
| {{Cirrhosis}} | | {{Cirrhosis}} |
| {{CMG}} {{AE}} | | {{CMG}} {{AE}} |
| == Gastric outlet obstruction==
| |
| GASTRIC OUTLET OBSTRUCTION: Pyloric obstruction
| |
|
| |
|
| Gastric outlet obstruction (GOO,) is the result of any pathology that provides mechanical obstruction to emptying of gastric contents.
| |
| Two important causes of GOO include:
| |
| Benign: 37 percent of cases, includes peptic disease
| |
| Malignant: 53 percent of cases
| |
|
| |
|
| Anatomy and pathophysiology
| | ===Pathophysiology prev=== |
| | <div style="-webkit-user-select: none;"> |
| | {| class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right;" |
| | |- |
| | | {{#ev:youtube|https://https://www.youtube.com/watch?v=5szNmKtyBW4|350}} |
| | |- |
| | |} |
| | __NOTOC__ |
| | {{Cirrhosis}} |
| | {{CMG}} {{AE}} |
|
| |
|
| Location of the stomach: Left upper quadrant of the abdomen
| | == History and Symptoms == |
| Parts of the stomach:
| |
| Cardia
| |
| Body
| |
| Antrum
| |
| Pylorus
| |
|
| |
|
| | * History should include: |
| | ** Appearance of bowel movements |
| | ** Travel history |
| | ** Associated symptoms |
| | ** Immune status |
| | ** Woodland exposure |
| | ==References== |
| | {{reflist|2}} |
|
| |
|
| In GOO, the antrum and pylous are mainly involved. Infiltration, scar formation or inflammation of these structures leads to GOO.
| | {{WH}} |
| The pylorus extends into the duodenum and is surrounded by the liver, gallbladder and pancreas. Malignancy of any of these neighbouring structures may lead to obstruction of the gastric outlet.
| | {{WS}} |
|
| |
|
| Clinical presentation:
| | ==Other Imaging Findings== |
| Vomitting: characteristic feature
| | * [[Endoscopy]] |
| Intolerance to solids, followed by liquids
| | * [[Barium enema]] |
| Dehydration
| | * [[Colonoscopy]] |
| Electrolyte abnormalities
| | * [[Sigmoidoscopy]] |
|
| |
|
| Late stages:
| | ==Other diagnostic studies== |
| Weight loss
| | == Other Diagnostic Studies == |
| Malnutrition: more pronounced in patients with malignancy
| |
| Abdominal distension
| |
| Aspiration pneumonia: due to dilatation of stomach, loss of contractility and accumulation of undigested food contents
| |
|
| |
|
| Etiology
| | * Breath hydrogen test |
| Benign causes:
| |
|
| |
|
| Acquired:
| | * [[HIV test]]ing for those patients suspected of having HIV |
| PUD:
| |
| 5 % cases ( most commonly affecting pylorus and initial part of the duodenum):
| |
| Acute- edema and inflammation
| |
| Chronic- due to intrinsic obstruction as a result of fibrosis and scar formation
| |
| Gastric polyps
| |
| Caustic ingestion
| |
| Obstruction by gallstones (Bouveret syndrome)
| |
| Complication of acute pancreatitis: pancreatic pseudocyst formation
| |
| bezoars
| |
|
| |
|
| Congenital:
| | == |
| Pyloric stenosis: most common cause in children
| |
| more common in boys> girls
| |
| due to hypertrophy of pyloric circular smooth muscles
| |
| Congenital duodenal webs
| |
|
| |
|
| Malignant causes-
| | ==Overview== |
| Malignancies involving neighbouring structures:
| |
| Pancreas: Pancreatic cancer: most common malignancy leading to extrinsic obstruction of the pylorus, occurs in one fifth of patients
| |
| Stomach: Gastric cancer
| |
| Duodenum: Duodenal cancer
| |
| Ampullary cancer
| |
| Bile duct: Cholangiocarcinomas
| |
| Secondary metastases to the gastric outlet by other primaries
| |
|
| |
|
| Epidemiology
| | ==References== |
| Incidence: less than 5% in patients with PUD.
| | {{reflist|2}} |
| PUD is the most common benign cause of GOO.
| |
| In the US, five percent PUD cases require an average of 2000 surgeries annually.
| |
| Pancreatic cancer is the most common malignant cause of GOO
| |
| Incidence of GOO in cases with pancreatic cancer is approximately 20%.
| |
|
| |
|
| ==Notes==
| | {{WH}} |
| Gastric outlet obstruction (GOO, also known as pyloric obstruction) is not a single entity; it is the clinical and pathophysiological consequence of any disease process that produces a mechanical impediment to gastric emptying.
| | {{WS}} |
| Clinical entities that can result in GOO generally are categorized into two well-defined groups of causes: benign and malignant. This classification facilitates discussion of management and treatment. In the past, when peptic ulcer disease (PUD) was more prevalent, benign causes were the most common; however, one review showed that only 37% of patients with GOO have benign disease and the remaining patients have obstruction secondary to malignancy.[1]
| |
| Gastric outlet obstruction can be a diagnostic and treatment dilemma. Despite medical advances in the acid suppression mechanism, the incidence of GOO remains a prevalent clinical problem in benign PUD. Also, an increase in the number of cases of GOO seems to be noted secondary to malignancy; this is possibly due to improvements in cancer therapy, which allow patients to live long enough to develop this complication.
| |
| As part of the initial workup, exclude the possibility of functional nonmechanical causes of obstruction, such as diabetic gastroparesis. Once a mechanical obstruction is confirmed, differentiate between benign and malignant processes because definitive treatment is based on recognition of the specific underlying cause.
| |
| Carry out diagnosis and treatment expeditiously, because delay may result in further compromise of the patient's nutritional status. Delay will also further compromise edematous tissue and complicate surgical intervention. Orient initial management to identification of the primary underlying cause and to the correction of volume and electrolyte abnormalities. Barium swallow studies and upper endoscopy are the main tests used to help make the diagnosis. Tailor treatment to the specific cause.
| |
|
| |
|
| Anatomy
| | ===Pathophysiology prev=== |
| The stomach is located mainly in the left upper quadrant beneath the diaphragm and is attached superiorly to the esophagus and distally to the duodenum. The stomach is divided into four portions: cardia, body, antrum, and pylorus. Inflammation, scarring, or infiltration of the antrum and pylorus are associated with the development of GOO.
| | <div style="-webkit-user-select: none;"> |
| The duodenum begins immediately beyond the pylorus and mostly is a retroperitoneal structure, wrapping around the head of the pancreas. The duodenum classically is divided into four portions. It is intimately related to the gallbladder, liver, and pancreas; therefore, a malignant process of any adjacent structure may cause outlet obstruction due to extrinsic compression.
| | {| class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right;" |
| Pathophysiology
| | |- |
| Intrinsic or extrinsic obstruction of the pyloric channel or duodenum is the usual pathophysiology of GOO; the mechanism of obstruction depends upon the underlying etiology.
| | | {{#ev:youtube|https://https://www.youtube.com/watch?v=5szNmKtyBW4|350}} |
| Patients present with intermittent symptoms that progress until obstruction is complete. Vomiting is the cardinal symptom. Initially, patients may demonstrate better tolerance to liquids than solid food. In a later stage, patients may develop significant weight loss due to poor caloric intake. Malnutrition is a late sign, but it may be very profound in patients with concomitant malignancy. In the acute or chronic phase of obstruction, continuous vomiting may lead to dehydration and electrolyte abnormalities.
| | |- |
| When obstruction persists, patients may develop significant and progressive gastric dilatation. The stomach eventually loses its contractility. Undigested food accumulates and may represent a constant risk for aspiration pneumonia.
| | |} |
| Etiology
| | __NOTOC__ |
| The major benign causes of GOO are PUD, gastric polyps, ingestion of caustics, pyloric stenosis, congenital duodenal webs, gallstone obstruction (Bouveret syndrome), pancreatic pseudocysts, and bezoars.
| | {{Cirrhosis}} |
| PUD manifests in approximately 5% of all patients with GOO. Ulcers within the pyloric channel and first portion of the duodenum usually are responsible for outlet obstruction. Obstruction can occur in an acute setting secondary to acute inflammation and edema or, more commonly, in a chronic setting secondary to scarring and fibrosis. Helicobacter pylori has been implicated as a frequent associated finding in patients with GOO, but its exact incidence has not been defined precisely.
| | {{CMG}} {{AE}} |
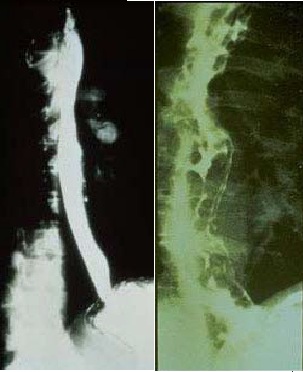
| Within the pediatric population, pyloric stenosis constitutes the most important cause of GOO. Pyloric stenosis occurs in 1 per 750 births. It is more common in boys than in girls and also is more common in first-born children. Pyloric stenosis is the result of gradual hypertrophy of the circular smooth muscle of the pylorus. (See image below.)
| |
| Anatomic changes associated with pyloric stenosis.
| |
| View Media Gallery
| |
| Pancreatic cancer is the most common malignancy causing GOO. Outlet obstruction may occur in 10-20% of patients with pancreatic carcinoma. Other tumors that may obstruct the gastric outlet include ampullary cancer, duodenal cancer, cholangiocarcinomas, and gastric cancer. Metastases to the gastric outlet also may be caused by other primary tumors.
| |
| Physical Examination
| |
| Physical examination often demonstrates the presence of chronic dehydration and malnutrition. A dilated stomach may be appreciated as a tympanitic mass in the epigastric area and/or left upper quadrant.
| |
| | |
| Dehydration and electrolyte abnormalities can be demonstrated by routine laboratory examinations. Increases in blood urea nitrogen (BUN) and creatinine are late features of dehydration.
| |
| | |
| Prolonged vomiting causes loss of hydrochloric acid and produces an increase of bicarbonate in the plasma to compensate for the lost chloride and sodium. The result is a hypokalemic hypochloremic metabolic alkalosis. Alkalosis shifts the intracellular potassium to the extracellular compartment, and the serum positive potassium is increased factitiously. With continued vomiting, the renal excretion of potassium increases in order to preserve sodium. The adrenocortical response to hypovolemia intensifies the exchange of potassium for sodium at the distal tubule, with subsequent aggravation of the hypokalemia
| |
| Physical Examination
| |
| Physical examination often demonstrates the presence of chronic dehydration and malnutrition. A dilated stomach may be appreciated as a tympanitic mass in the epigastric area and/or left upper quadrant.
| |
| Dehydration and electrolyte abnormalities can be demonstrated by routine laboratory examinations. Increases in blood urea nitrogen (BUN) and creatinine are late features of dehydration.
| |
| Prolonged vomiting causes loss of hydrochloric acid and produces an increase of bicarbonate in the plasma to compensate for the lost chloride and sodium. The result is a hypokalemic hypochloremic metabolic alkalosis. Alkalosis shifts the intracellular potassium to the extracellular compartment, and the serum positive potassium is increased factitiously. With continued vomiting, the renal excretion of potassium increases in order to preserve sodium. The adrenocortical response to hypovolemia intensifies the exchange of potassium for sodium at the distal tubule, with subsequent aggravation of the hypokalemia
| |
| Once the diagnosis of gastric outlet obstruction (GOO) is suspected, request a surgical consultation. GOO due to benign ulcer disease may be treated medically if results of imaging studies or endoscopy determine that acute inflammation and edema are the principal causes of the outlet obstruction (as opposed to scarring and fibrosis, which may be fixed).
| |
| | |
| If medical therapy conducted for a reasonable period fails to alleviate the obstruction, then surgical intervention becomes appropriate. Typically, if resolution or improvement is not seen within 48-72 hours, surgical intervention is necessary. The choice of surgical procedure depends upon the patient's particular circumstances; however, vagotomy and antrectomy should be considered the criterion standard against which the efficacy of other procedures is measured.
| |
| | |
| Laboratory Studies
| |
| Obtain a complete blood count (CBC). Check the hemoglobin and hematocrit to rule out the possibility of anemia. Obtain an electrolyte panel. As noted previously, identifying and correcting electrolyte abnormalities that tend to occur is essential. Liver function tests may be helpful, particularly when a malignant etiology is suspected. A test for H pylori is helpful when the diagnosis of peptic ulcer disease (PUD) is suspected.
| |
| | |
| Imaging Studies
| |
| Plain abdominal radiography, contrast upper gastrointestinal (GI) studies (Gastrografin or barium), and computed tomography (CT) with oral contrast are helpful. (See the images below.) Plain radiographs, including the obstruction series (ie, supine abdomen, upright abdomen, chest posteroanterior), can demonstrate the presence of gastric dilatation and may be helpful in distinguishing the differential diagnosis.
| |
| | |
| Diagnostic Procedures
| |
| Upper endoscopy (see the image below) can help visualize the gastric outlet and may provide a tissue diagnosis when the obstruction is intraluminal.
| |
| The sodium chloride load test is a traditional clinical nonimaging study that may be helpful. The traditional sodium chloride load test is performed by infusing 750 mL of sodium chloride solution into the stomach via a nasogastric tube (NGT). A diagnosis of gastric outlet obstruction (GOO) is made if more than 400 mL remains in the stomach after 30 minutes.
| |
| Nuclear gastric emptying studies measure the passage of orally administered radionuclide over time. Unfortunately, both the nuclear test and the saline load test may produce abnormal results in functional states.
| |
| Barium upper GI studies are very helpful because they can delineate the gastric silhouette and demonstrate the site of obstruction. An enlarged stomach with a narrowing of the pyloric channel or first portion of the duodenum helps differentiate GOO from gastroparesis.
| |
| The specific cause may be identified as an ulcer mass or intrinsic tumor.
| |
| In the presence of PUD, perform endoscopic biopsy to rule out the presence of malignancy. In the case of peripancreatic malignancy, CT-guided biopsy may be helpful in establishing a preoperative diagnosis. Needle-guided biopsy also may be helpful in establishing the presence of metastatic disease. This knowledge may impact the magnitude of the procedure planned to alleviate the GOO. Histologic findings relate to the individual underlying cause.
| |
| Laboratory Studies
| |
| Obtain a complete blood count (CBC). Check the hemoglobin and hematocrit to rule out the possibility of anemia. Obtain an electrolyte panel. As noted previously, identifying and correcting electrolyte abnormalities that tend to occur is essential. Liver function tests may be helpful, particularly when a malignant etiology is suspected. A test for H pylori is helpful when the diagnosis of peptic ulcer disease (PUD) is suspected.
| |
| The sodium chloride load test is a traditional clinical nonimaging study that may be helpful. The traditional sodium chloride load test is performed by infusing 750 mL of sodium chloride solution into the stomach via a nasogastric tube (NGT). A diagnosis of gastric outlet obstruction (GOO) is made if more than 400 mL remains in the stomach after 30 minutes.
| |
| | |
| Nuclear gastric emptying studies measure the passage of orally administered radionuclide over time. Unfortunately, both the nuclear test and the saline load test may produce abnormal results in functional states.
| |
| | |
| Barium upper GI studies are very helpful because they can delineate the gastric silhouette and demonstrate the site of obstruction. An enlarged stomach with a narrowing of the pyloric channel or first portion of the duodenum helps differentiate GOO from gastroparesis.
| |
| | |
| The specific cause may be identified as an ulcer mass or intrinsic tumor.
| |
| | |
| In the presence of PUD, perform endoscopic biopsy to rule out the presence of malignancy. In the case of peripancreatic malignancy, CT-guided biopsy may be helpful in establishing a preoperative diagnosis. Needle-guided biopsy also may be helpful in establishing the presence of metastatic disease. This knowledge may impact the magnitude of the procedure planned to alleviate the GOO. Histologic findings relate to the individual underlying cause.
| |
| | |
| Once the diagnosis of gastric outlet obstruction (GOO) is suspected, request a surgical consultation. GOO due to benign ulcer disease may be treated medically if results of imaging studies or endoscopy determine that acute inflammation and edema are the principal causes of the outlet obstruction (as opposed to scarring and fibrosis, which may be fixed).
| |
| | |
| If medical therapy conducted for a reasonable period fails to alleviate the obstruction, then surgical intervention becomes appropriate. Typically, if resolution or improvement is not seen within 48-72 hours, surgical intervention is necessary. The choice of surgical procedure depends upon the patient's particular circumstances; however, vagotomy and antrectomy should be considered the criterion standard against which the efficacy of other procedures is measured.
| |
| | |
| In cases of malignant obstruction, weigh the extent of surgical intervention for the relief of GOO against the malignancy's type and extent, as well as the patient's anticipated long-term prognosis. As a guiding principle, undertake major tumor resections in the absence of metastatic disease in a patient who can withstand such a procedure from a nutritional standpoint. In patients with largely metastatic disease, determine the degree of surgical intervention for palliation in light of the patient's realistic prognosis and personal wishes.
| |
| | |
| Contraindications for surgery relate to the underlying medical condition. Most patients benefit from an initial period of gastric decompression, hydration, and correction of electrolyte imbalances. In patients who are severely malnourished, postponing surgical intervention until the nutritional status has been optimized may be wise. In selective cases, some patients may benefit from total parenteral nutrition (TPN) or distal tube feeding (eg, placed via a percutaneous jejunostomy).
| |
| | |
| One of the relative contraindications for surgery is the presence of advanced malignancy; in these cases, in which life expectancy may be limited to a few months, palliation via endoscopically placed stents should be considered.
| |
| | |
| Overall, every patient with GOO deserves evaluation by a surgeon. Even if the patient has unresectable disease, palliative surgical measures may improve the quality of life.
| |
| | |
| Initial management of GOO should be the same regardless of the primary cause. After a diagnosis is made, admit patients for hydration and correction of electrolyte abnormalities. Remembering that the metabolic alkalosis of GOO responds to the administration of chloride is important; therefore, sodium chloride solution should be the initial intravenous (IV) fluid of choice. Potassium deficits are corrected after repletion of volume status and after replacement of chloride.
| |
| | |
| Place a nasogastric (NG) tube to decompress the stomach. Occasionally, a large tube is required because the undigested food blocks tubes with small diameters.
| |
| | |
| When acute peptic ulcer disease (PUD) has been identified as a primary cause of GOO, focus treatment on the reduction of acid production. Histamine-2 (H2) blockers and proton pump inhibitors are the mainstay of treatment.
| |
| | |
| Treat ''H pylori'' infection, when identified, according to current recommendations. Although most patients improve temporarily with treatment, scarring and fibrosis may worsen over time. Pneumatic balloon dilatation of a chronic, benign stricture can be performed via endoscopy. Patients who are candidates for balloon dilatation are likely to present with recurrent GOO.
| |
| | |
| Published series using this technique report success rates of over 76% after multiple dilatations,<sup> [[null 3]] </sup> though the rate of failure and recurrent obstruction is higher in patients treated with balloon dilatation who have not also been treated for ''H pylori'' infection.<sup> [[null 4]] </sup>Patients who are negative for ''H pylori'' do not respond favorably to balloon dilatation and should be considered for surgical treatment early in the process.<sup> [[null 5]]</sup>
| |
| | |
| Further treatment is tailored to the underlying cause; this is where the distinction between benign and malignant disease becomes important.
| |
| | |
| === Preparation for surgery === | |
| Perform standard preoperative evaluation in these patients. Correct fluid and electrolyte abnormalities prior to surgery. Perform gastric decompression by NG tube and suction and alert the anesthesiologist to the potential risk for aspiration upon induction.
| |
| | |
| Perform a preoperative nutritional evaluation and initiate appropriate nutritional therapy (ie, TPN or enteral feedings via a percutaneous jejunostomy placed distal to the obstruction) as soon as possible. Maximizing preoperative nutrition can greatly reduce or eliminate postoperative complications related to delayed healing.
| |
| | |
| === Management of benign disease ===
| |
| More than 75% of patients presenting with GOO eventually require surgical intervention.<sup> [[null 6]] </sup>Surgical intervention usually provides definitive treatment of GOO, but it may result in its own comorbid consequences. Operative management should offer relief of obstruction and correction of the acid problem.
| |
| | |
| The most common surgical procedures performed for GOO related to PUD are vagotomy and antrectomy, vagotomy and pyloroplasty, truncal vagotomy and gastrojejunostomy, pyloroplasty, and laparoscopic variants<sup> [[null 7]] </sup>of the aforementioned procedures. The video below demonstrates robotic-assisted pyloroplasty.
| |
| | |
| Vagotomy and antrectomy with Billroth II reconstruction (gastrojejunostomy) seem to offer the best results. Vagotomy and pyloroplasty and pyloroplasty alone, although used with some success, can be technically difficult to perform due to scarring at the gastric outlet. A combination of balloon dilatation and highly selective vagotomy has been described, but it is associated with gastroparesis and a high recurrence rate.
| |
| | |
| Placement of a jejunostomy tube at the time of surgery should be considered. This provides temporary feeding access in already malnourished patients. Also, in chronically dilated partial obstructions, the stomach may be slow to recover a normal rate of emptying.
| |
| | |
| The role of the laparoscopic approach in the treatment of GOO is under investigation and may represent a valid form of therapy with low morbidity. The experience of several international centers has been published. One group in China performed laparoscopic truncal vagotomy and gastrojejunostomy for GOO related to PUD, with nearly complete resolution of symptomatology. The investigators reported no conversions to open procedure or mortalities. Twenty-seven percent of patients did experience transiently delayed gastric emptying, which resolved with conservative measures.<sup> [[null 8]]</sup>
| |
| | |
| Kim et al also reported good results from the use of laparoscopic truncal vagotomy with gastrojejunostomy, including shorter operating times and hospital stays in comparison with the open procedure.<sup> [[null 9]]</sup>
| |
| | |
| Hall et al performed a double-blind, multicenter, randomized, controlled trial comparing patient recovery following laparoscopic pyloromyotomy to that after open pyloromyotomy in infants with pyloric stenosis.<sup> [[null 10]] </sup>(See image below.) The investigators found that among the 87 infants who underwent the laparoscopic procedure, the median (interquartile range) postoperative time needed to achieve full enteral feeding was 18.5 hours, compared with 23.9 hours in the 93 infants who underwent open pyloromyotomy. The median postoperative length of stay in the laparoscopic and open pyloromyotomy groups were 33.6 hours and 43.8 hours, respectively.
| |
| The study also found that the incidence of postoperative vomiting was similar in the open and laparoscopic groups, as was the frequency of intraoperative and postoperative complications. The authors suggested that open and laparoscopic pyloromyotomy are safe means of treating infantile pyloric stenosis. Because of its apparent advantages, however, they recommended that in centers with suitable laparoscopic experience, the laparoscopic form of the procedure be used.
| |
| | |
| Management of malignant disease
| |
| The management of GOO secondary to malignancy is controversial. Of patients with periampullary cancer, 30-50% present with nausea and vomiting at the time of diagnosis. [11, 12] Most of these tumors are unresectable (approximately 40% of gastric cancers and 80-90% of periampullary cancers). [13, 14] When tumors are found to be unresectable, 13-20% of patients eventually develop GOO before they succumb to their disease. The 1-year survival rate is poor.
| |
| | |
| Gastrojejunostomy remains the surgical treatment of choice for GOO secondary to malignancy. Although surgeons traditionally have preferred an antecolic anastomosis to prevent further obstruction by advancing tumor growth, a publication evaluating the retrocolic anastomosis in this setting challenges conventional wisdom. [15] Results demonstrate that a retrocolic anastomosis may be associated with decreased incidences of delayed gastric emptying (6% vs 17%) and late GOO (2% vs 9%).
| |
| | |
| Other groups have illustrated that partial stomach-partitioning gastrojejunostomy decreases the rates of delayed gastric emptying as compared with traditional gastrojejunostomy. [16] Feeding jejunostomy should again be considered to combat malnutrition and slow recovery of gastric emptying.
| |
| | |
| Internationally, studies are under way using laparoscopic gastrojejunostomy instead of the open procedure. In the United States, critics cite a nearly 20% conversion rate and a delay in the return of gut function as reasons to not perform the procedure laparoscopically. Comparisons of laparoscopic GI anastomosis versus the open procedure have revealed less morbidity and mortality, shorter hospital stays, fewer blood transfusions, and faster GI transit recovery time. [17, 18]
| |
| | |
| Researchers at Johns Hopkins Hospital have attempted endoscopic transgastric approaches to create a gastrojejunostomy in a porcine model. [19] As natural orifice transluminal surgery gains more widespread interest, these novel approaches may become more popular.
| |
| | |
| Chopita et al reported on the use of magnetic endoscopic gastroenteric anastomosis in 15 patients with malignant gastroenteric obstruction. The procedure had an 86.7% success rate, with the authors noting the additional benefits of shorter duration of hospital stay and good quality of life in patients. [20] Although still experimental, this procedure may one day be a surgical option.
| |
| | |
| No et al reported that gastrojejunostomy was preferable to metal stent placement in providing palliation of GOO caused by unresectable or metastatic cancer in patients with a good performance status. [21]
| |
| | |
| Self-expandable metallic stents also have been used for the treatment of GOO in a malignant setting. [22, 23] Metallic stents have previously been used successfully to treat stenosis of such areas as the blood vessels, bile duct, esophagus, and trachea. With the development of newer stents and delivery systems, metallic stents may have a role in the nonsurgical treatment of gastroduodenal obstruction. Stents may allow the physician to avoid complicated surgical procedures.
| |
| | |
| Currently, only the Wallstent has FDA approval for palliation in malignant gastroduodenal obstruction. Significant complications include the following: malposition, misdeployment, tumor ingrowth or overgrowth, migration, bleeding, and perforation. [24]
| |
| | |
| A review of 19 studies published in 2004 quoted clinical success rates of 80-90%. [25] Subsequent multicenter trials using the enteral Wallstent in 176 patients with malignant GOO resulted in 89% of patients tolerating oral intake for a median of 219 days postprocedure. Of the 84% in whom the stent was successful after the initial procedure, 22% required restenting to tolerate an oral diet. In addition, as other studies have demonstrated, chemotherapy was independently associated with an increased tolerance in oral intake. [26]
| |
| | |
| One proposed solution uses covered metallic stents that have a lower incidence of tumor ingrowth. A 60% rate of tumor ingrowth in uncovered stents versus a 10% rate of tumor ingrowth in covered stents has been reported. Furthermore, with the double stent technique, that is, simultaneous placement of both covered stents and uncovered stents, lower early restenosis rates have been achieved. A stent patency of 21.5 days for uncovered stents versus 150 days for double stents has been achieved. [27]
| |
| | |
| Of the 62 patients studied by Maetani et al, half received uncovered stents and half covered stents. The authors found no statistical differency in patency, but the triple-covered stent resulted in less frequent dysfunction four weeks after stenting. [28]
| |
| | |
| Several retrospective studies have been performed to compare the results of stenting withm those of surgical intervention. Survival rates are equivalent; however, costs, length of stay, and number of subsequent procedures are all decreased following stenting. [29, 30] In addition, a delay of gastric emptying and morbidity decrease with the use of metallic stents. [31] These promising results suggest that stents may eventually replace surgery as palliative intervention for unresectable periampullary malignancies.
| |
| | |
| A 2011 study from the Netherlands discusses the use of a D-Weave Niti-S nitinol stent specifically for the duodenum. A key outcome in this palliative procedure was a significant improvement in global health status and median 82-day survival. The authors report a marvelous technical and robust clinical success rate with patency up to 190 days, and a 25% complication rate. [32]
| |
| | |
| In a 2014 study designed to compare the outcomes of endoscopic enteral stent insertion for malignant gastric outlet obstruction in older (≥65 years; n = 26) versus younger (n = 56) patients, Mansoor and Zeb reported comparable rates of technical success (100% in older patients vs 97% in younger patients) and clinical success in the two groups. [33] The complication rates were also comparable (27% in older patients vs 23% in younger patients).
| |
| | |
| The role of prophylactic gastrojejunostomy in cases of malignant GOO is a question that remains to be answered. Some surgeons argue that prophylactic gastrojejunostomy may increase postoperative morbidity, primarily due to delayed gastric emptying.
| |
| | |
| This issue was addressed in a study by Lillemoe and Cameron, [15] in which 87 patients with unresectable periampullary cancer were randomized to receive or not receive a prophylactic gastrojejunostomy. Although there were no significant differences in morbidity, length of hospital stay, and survival rates, the prophylactic gastrojejunostomy group had a 0% (0/44) incidence of GOO versus 19% (8/43) in the other group. The authors concluded that prophylactic gastrojejunostomy significantly decreased the incidence of late GOO and should be performed routinely when a patient is undergoing surgical palliation for periampullary cancer. Comparable data are supported by other series.
| |
| Perform standard preoperative evaluation in these patients. Correct fluid and electrolyte abnormalities prior to surgery. Perform gastric decompression by NG tube and suction and alert the anesthesiologist to the potential risk for aspiration upon induction.
| |
| | |
| Perform a preoperative nutritional evaluation and initiate appropriate nutritional therapy (ie, TPN or enteral feedings via a percutaneous jejunostomy placed distal to the obstruction) as soon as possible. Maximizing preoperative nutrition can greatly reduce or eliminate postoperative complications related to delayed healing.
| |
| | |
| Management of benign disease
| |
| More than 75% of patients presenting with GOO eventually require surgical intervention. [6] Surgical intervention usually provides definitive treatment of GOO, but it may result in its own comorbid consequences. Operative management should offer relief of obstruction and correction of the acid problem.
| |
| | |
| The most common surgical procedures performed for GOO related to PUD are vagotomy and antrectomy, vagotomy and pyloroplasty, truncal vagotomy and gastrojejunostomy, pyloroplasty, and laparoscopic variants [7] of the aforementioned procedures. The video below demonstrates robotic-assisted pyloroplasty.
| |
| Admit patients to a monitor unit after the procedure. Pay special attention to fluid and electrolyte status.
| |
| | |
| Most surgeons agree that perioperative antibiotics are advisable but may be limited to use during the immediate perioperative period in the absence of intervening infection.
| |
| | |
| If a gastric reconstruction is performed, NG intubation is recommended. The length of time that the tube should remain in place is controversial; however, it is important to remember that a previously dilated stomach, the performance of a vagotomy, and the presence of metastatic cancer may all contribute to decreased gastric motility. An anatomically patent gastrojejunostomy may fail to empty for days. This syndrome of delayed gastric emptying is a well-known entity and requires surgical patience. Again, preoperative planning for feeding access becomes important during this immediate postoperative period.
| |
| | |
| Aggressive pulmonary toilet, prophylaxis for gastritis and deep venous thrombosis (DVT), and early ambulation are advisable.
| |
|
| |
|
| ==Video codes== | | ==Video codes== |
| Line 239: |
Line 87: |
| {{#ev:youtube|4uSSvD1BAHg}} | | {{#ev:youtube|4uSSvD1BAHg}} |
| {{#ev:youtube|PQXb5D-5UZw}} | | {{#ev:youtube|PQXb5D-5UZw}} |
| | {{#ev:youtube|UVJYQlUm2A8}} |
|
| |
|
| ===Video in table=== | | ===Video in table=== |
| Line 270: |
Line 119: |
| ===Image and text to the right=== | | ===Image and text to the right=== |
|
| |
|
| <figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline>[[File:Global distribution of leptospirosis.jpg|577x577px]]</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017. | | <figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline>[[File:Global distribution of leptospirosis.jpg|577x577px]]</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017. |
|
| |
|
| ===Gallery=== | | ===Gallery=== |
| Line 285: |
Line 134: |
| ==References== | | ==References== |
| {{Reflist|2}} | | {{Reflist|2}} |
|
| |
| [[Category:Gastroenterology]]
| |
| [[Category:Hepatology]]
| |
| [[Category:Disease]]
| |
|
| |
| {{WS}} | | {{WS}} |
| {{WH}} | | {{WH}} |
| Line 296: |
Line 140: |
| REFERENCES | | REFERENCES |
| <references /> | | <references /> |
| | |
| | [[Category:Gastroenterology]] |
| | [[Category:Needs overview]] |
| | [[Category:Hepatology]] |
| | [[Category:Disease]] |
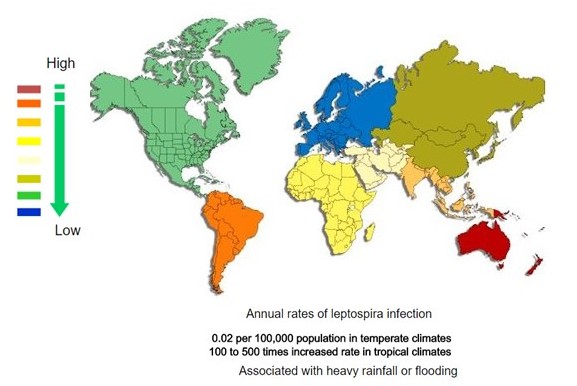 </figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)