Oesophagostomum pathophysiology: Difference between revisions
m (Changes made per Mahshid's request) |
|||
| (7 intermediate revisions by one other user not shown) | |||
| Line 11: | Line 11: | ||
Single-nodular disease, more commonly known as Dapaong disease, is characterized by the development of a single mass that develops throughout the colon wall. This is the most common form of oesophagostomiasis, affecting 85% of patients.<ref>“GIDEON Infectious Diseases - Diseases.” GIDEON Infectious Disease Database. 5 Feb 2009.<http://web.gideononline.com/web/epidemiology/index.php?gdn_form=ZGlzZWFzZT0xMTY1MA==>.</ref> This nodule can instigate intense tissue reactions that result in the formation of painful projecting masses. | Single-nodular disease, more commonly known as Dapaong disease, is characterized by the development of a single mass that develops throughout the colon wall. This is the most common form of oesophagostomiasis, affecting 85% of patients.<ref>“GIDEON Infectious Diseases - Diseases.” GIDEON Infectious Disease Database. 5 Feb 2009.<http://web.gideononline.com/web/epidemiology/index.php?gdn_form=ZGlzZWFzZT0xMTY1MA==>.</ref> This nodule can instigate intense tissue reactions that result in the formation of painful projecting masses. | ||
===Life Cycle=== | ===Life Cycle=== | ||
[[Image:Oesophagostomum LifeCycle.gif|thumb|center|500px|Common livestock such as sheep, goats, and swine, as well as non-human primates, are the usual definitive hosts for ''Oesophagostomum'' spp., but other animals, including humans and cattle, may also serve as definitive hosts. Eggs are shed in the feces of the definitive host (1), and may be indistinguishable from the eggs of ''Necator'' and ''Ancylostoma''. Eggs hatch into rhabditiform (L1) larvae in the environment (2), given appropriate temperature and level of humidity. In the environment, the larvae will undergo two molts and become infective filariform (L3) larvae (3). Worms can go from eggs to L3 larvae in a matter of a few days, given appropriate environmental conditions. Definitive hosts become infected after ingesting infective L3 larvae (4). After ingestion, L3 larvae burrow into the submucosa of the large or small intestine and induce cysts. Within these cysts, the larvae molt and become L4 larvae. These L4 larvae migrate back to the lumen of the large intestine, where they molt into adults (5). Eggs appear in the feces of the definitive host about a month after ingestion of infective L3 larvae.]] | |||
For non-human hosts, the life cycle of ''Oesophagostomum'' begins with the passing of eggs in the animal feces. From there the eggs develop into stage one larvae. These larvae then spend 6–7 days in the environment developing into stage two and then infectious stage three larvae.<ref>Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.</ref> Infection begins with the ingestion of soil contaminated with stage three larvae. After ingestion the larvae end up in the small intestine, unsheathing and penetrating the intestinal wall to form nodules. The resulting adult worms that remain in the intestinal lumen copulate; the eggs from the female are then deposited in the feces. Females usually lay around 5,000 eggs per day, which is on par with reproductive rates of other nematodes within Strongyloidea.<ref name="Krepel, H P 1992">Krepel, H P, and A M Polderman. “Egg production of Oesophagostomum bifurcum, a locally common parasite of humans in Togo.” The American Journal of Tropical Medicine and Hygiene 46.4 (1992): 469-72.</ref> | For non-human hosts, the life cycle of ''Oesophagostomum'' begins with the passing of eggs in the animal feces. From there the eggs develop into stage one larvae. These larvae then spend 6–7 days in the environment developing into stage two and then infectious stage three larvae.<ref>Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.</ref> Infection begins with the ingestion of soil contaminated with stage three larvae. After ingestion the larvae end up in the small intestine, unsheathing and penetrating the intestinal wall to form nodules. The resulting adult worms that remain in the intestinal lumen copulate; the eggs from the female are then deposited in the feces. Females usually lay around 5,000 eggs per day, which is on par with reproductive rates of other nematodes within Strongyloidea.<ref name="Krepel, H P 1992">Krepel, H P, and A M Polderman. “Egg production of Oesophagostomum bifurcum, a locally common parasite of humans in Togo.” The American Journal of Tropical Medicine and Hygiene 46.4 (1992): 469-72.</ref> | ||
| Line 18: | Line 20: | ||
====Eggs==== | ====Eggs==== | ||
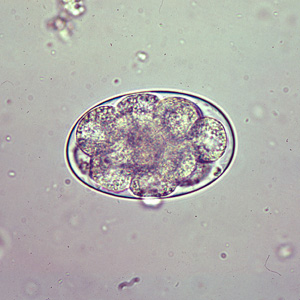
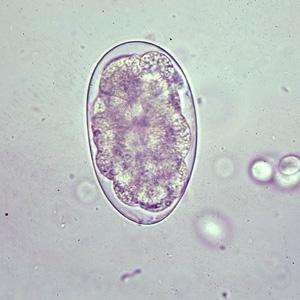
Eggs of Oesophagostomum bifurcum, the most-common species infecting humans, cannot be differentiated morphologically from the eggs of Necator or Ancylostoma (eggs of other animal oesophagostome species tend to be larger than typical hookworm eggs, however). The eggs of O. bifurcum measure 60-75 µm long by 35-40 µm wide. Eggs are often in a later stage of cleavage than hookworm species when shed in feces. | Eggs of Oesophagostomum bifurcum, the most-common species infecting humans, cannot be differentiated morphologically from the eggs of Necator or Ancylostoma (eggs of other animal oesophagostome species tend to be larger than typical hookworm eggs, however). The eggs of O. bifurcum measure 60-75 µm long by 35-40 µm wide. Eggs are often in a later stage of cleavage than hookworm species when shed in feces. | ||
<div align="center"> | |||
<gallery heights="175" widths="175"> | |||
Image:Oesophagostomum egg A.jpg|Egg of Oesophagostomum sp.in an unstained wet mount of stool. | |||
Image:Oesophagostomum egg B.jpg|Egg of Oesophagostomum sp.in an unstained wet mount of stool. | |||
</gallery> | |||
</div> | |||
Eggs are ovular in shape and range from 50 to 100 micrometres in size; they closely resembles those of hookworms, which renders diagnosis via stool analysis useless in areas co-infected with both ''Oesophagostomum'' and hookworm.<ref>Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.</ref> | Eggs are ovular in shape and range from 50 to 100 micrometres in size; they closely resembles those of hookworms, which renders diagnosis via stool analysis useless in areas co-infected with both ''Oesophagostomum'' and hookworm.<ref>Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.</ref> | ||
| Line 49: | Line 56: | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Nematodes]] | [[Category:Nematodes]] | ||
[[Category:Zoonoses]] | [[Category:Zoonoses]] | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
Latest revision as of 18:31, 18 September 2017
|
Oesophagostomum Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Oesophagostomum pathophysiology On the Web |
|
American Roentgen Ray Society Images of Oesophagostomum pathophysiology |
|
Risk calculators and risk factors for Oesophagostomum pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
In oesophagostomiasis, larvae can invade the colon wall, potentially causing two pervading types of nodular pathology. Multinodular disease is characterized by the formation of many tiny nodular lesions containing worms and pus along the colon wall. About 15% of patients have this form of oesophagostomiasis.[1]
Pathophysiology
Nodules themselves are usually not a problem, but they can give rise to further complications, such as bowel obstruction, peritonitis and intestinal volvulus. In rare cases serious disease can occur including emaciation, fluid in the pericardium, cardiomegaly, hepatosplenomegaly, perisplenitis, and enlargement of the appendix.
Single-nodular disease, more commonly known as Dapaong disease, is characterized by the development of a single mass that develops throughout the colon wall. This is the most common form of oesophagostomiasis, affecting 85% of patients.[2] This nodule can instigate intense tissue reactions that result in the formation of painful projecting masses.
Life Cycle
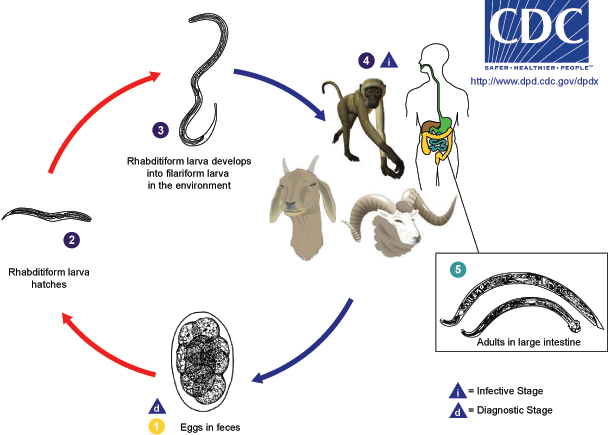
For non-human hosts, the life cycle of Oesophagostomum begins with the passing of eggs in the animal feces. From there the eggs develop into stage one larvae. These larvae then spend 6–7 days in the environment developing into stage two and then infectious stage three larvae.[3] Infection begins with the ingestion of soil contaminated with stage three larvae. After ingestion the larvae end up in the small intestine, unsheathing and penetrating the intestinal wall to form nodules. The resulting adult worms that remain in the intestinal lumen copulate; the eggs from the female are then deposited in the feces. Females usually lay around 5,000 eggs per day, which is on par with reproductive rates of other nematodes within Strongyloidea.[4]
For human hosts, the life cycle is very similar to that of Oesophagostomum in animals. It begins when an animal reservoir defecates into the soil, leaving feces infested with eggs that develop into rhabitiform larvae.[5] These larve then develop into stage two and then infectious stage three larvae in the environment over the course of 6–7 days. Human infection occurs when soil or water containing the third-stage larvae is ingested, presumably via contaminated meat obtained from infected livestock or crops with contaminated soil. Once ingested, the filariform larvae migrate to the submucosa of the small or large intestine, then to the lumen of the colon. The developing worms then penetrate the intestinal tissues, causing nodular lesion formation in the intestines and colon; it is in these nodules that the larvae mature to stage four larvae. These larvae may then emerge from their nodules and migrate back to the intestinal lumen, where they mature into adults. But many larvae often do not complete development and remain in their colon nodules, as humans are generally unsuitable hosts for Oesophagostomum. The instances whereOesophagostomum have completed development in humans seem to be dependent on certain environmental and host factors that have yet to be identified.[6]
Microscopic Pathology
Eggs
Eggs of Oesophagostomum bifurcum, the most-common species infecting humans, cannot be differentiated morphologically from the eggs of Necator or Ancylostoma (eggs of other animal oesophagostome species tend to be larger than typical hookworm eggs, however). The eggs of O. bifurcum measure 60-75 µm long by 35-40 µm wide. Eggs are often in a later stage of cleavage than hookworm species when shed in feces.
-
Egg of Oesophagostomum sp.in an unstained wet mount of stool.
-
Egg of Oesophagostomum sp.in an unstained wet mount of stool.
Eggs are ovular in shape and range from 50 to 100 micrometres in size; they closely resembles those of hookworms, which renders diagnosis via stool analysis useless in areas co-infected with both Oesophagostomum and hookworm.[7]
Adults
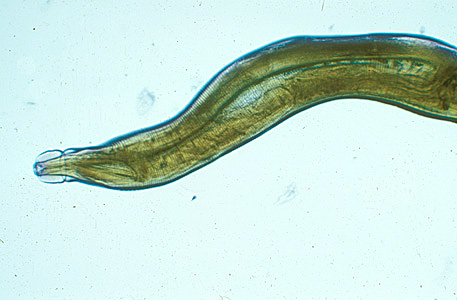
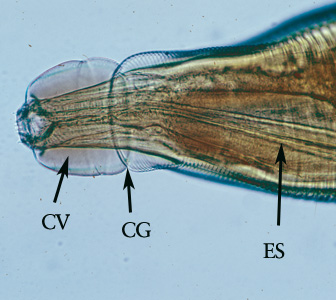
Adult worms of all Oesophagostomum spp. exhibit a cephalic groove by its proximal gut as well as a visible secretory pore, or stomum, at the same level of the oesophagus19. Like other nematodes, Oesophagostomum spp. contain a developed, multi-nucleate digestive tract as well as a reproductive system. Their developed buccal capsule and club-shaped oesophagus are useful for distinguishing Oesophagostomum spp. from hookworms.[8]
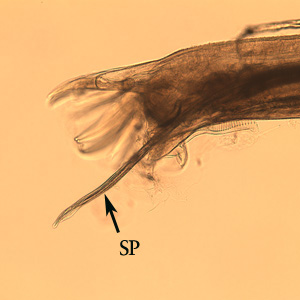
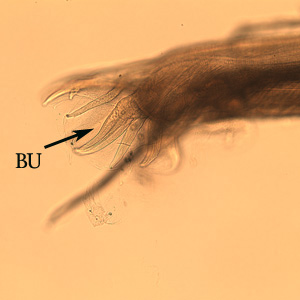
Both sexes of adults have a cephalic inflation and an oral opening lined with both internal and external leaf crowns. Female adults, which have a length range of 6.5–24 mm, are generally larger than their male counterparts, with a length range of 6-16.6 mm. Males can be distinguished by their bell-like copulatory bursa, located in the tail, and their paired rodlike spicules.[9]
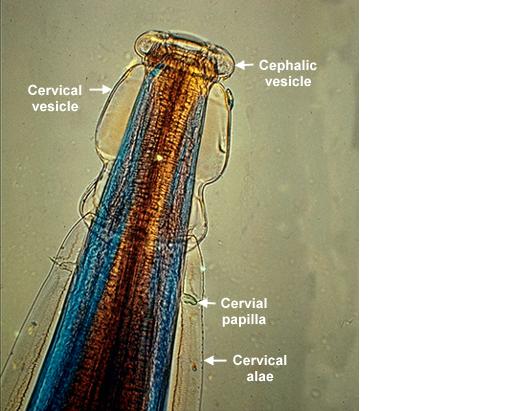
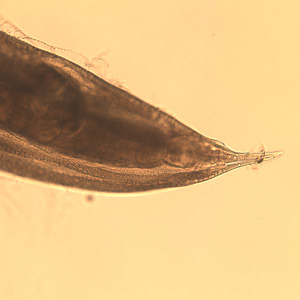
Adults of Oesophagostomum spp. are bursate nematodes, related to and morphologically-similar to, the hookworms. Females measure 1.5-3.0 cm in length; males are smaller. In both sexes, the anterior end has a cephalic inflation or vesicle, a transverse cephalic groove, and an oral opening guarded by external and internal leaf crowns (corona radiata). The cuticle is ringed with transverse striations. The posterior end of the female is short and pointed; the male possesses a symmetrical bursa and paired, equal spicules. Adults reside in the large intestine of the definitive host.
-
Adult of Oesophagostomum sp.
-
Higher magnification of the anterior end of the specimen. Note the presence of the cephalic vesicle (CV), cephalic groove (CG) and esophagus (ES).
-
Higher magnification of the anterior end of the specimen. Note the presence of the cephalic vesicle (CV) and corona radiata (CR).
-
Posterior end of a female Oesophagostomum sp., showing the pointed tail.
-
Posterior end of a male Oesophagostomum sp., Note the spicule (SP).
-
Posterior end of a male Oesophagostomum sp., Note the bursa (BU).
-
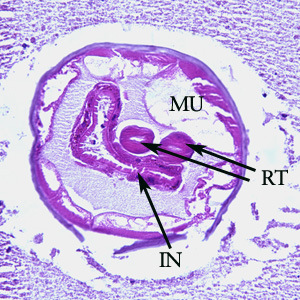
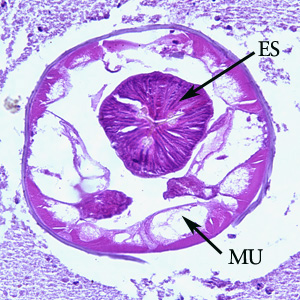
Cross-section of an adult of Oesophagostomum sp. in a colon biopsy specimen from a patient from Africa, stained with hematoxylin and eosin (H&E). Note the large, platymyarian muscle cells (MU), intestine with brush border (IN), paired reproductive tubes (RT). This image is taken at 200x magnification.
-
Cross-section of an adult of Oesophagostomum sp. in a colon biopsy specimen from a patient from Africa, stained with hematoxylin and eosin (H&E). Note the large, platymyarian muscle cells (MU) and thick, muscled esophagus (ES). Image taken at 200x magnification.
References
- ↑ “GIDEON Infectious Diseases - Diseases.” GIDEON Infectious Disease Database. 5 Feb 2009. <http://web.gideononline.com/web/epidemiology/index.php?gdn_form=ZGlzZWFzZT0xMTY1MA==>.
- ↑ “GIDEON Infectious Diseases - Diseases.” GIDEON Infectious Disease Database. 5 Feb 2009.<http://web.gideononline.com/web/epidemiology/index.php?gdn_form=ZGlzZWFzZT0xMTY1MA==>.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Krepel, H P, and A M Polderman. “Egg production of Oesophagostomum bifurcum, a locally common parasite of humans in Togo.” The American Journal of Tropical Medicine and Hygiene 46.4 (1992): 469-72.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Ziem, J.B. et al. “Impact of repeated mass treatment on human Oesophagostomum and hookworm infections in northern Ghana.” Tropical Medicine & International Health: TM & IH 11.11 (2006): 1764-72.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006.<https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.
- ↑ Elmes, B et al. (1953). Helminthic abscess, a surgical complication of oesophagostomes and hookworms. Annals of Tropical Medicine and Parasitology. 48: 1-7.
- ↑ Ziem, J.B. “Controlling human oesophagostomiasis in northern Ghana.” (Doctoral thesis) Leiden University. 2006. <https://openaccess.leidenuniv.nl/dspace/handle/1887/4917?mode=more>.