Quassia: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Quassia": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (4 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag=<!--Overview--> | |authorTag=<!--Overview-->{{RB}} | ||
|OTC=Yes | |||
|genericName=Quassia | |||
|aOrAn=a | |aOrAn=a | ||
|drugClass=OTC cream | |||
|indicationType=treatment | |indicationType=treatment | ||
| | |indication=[[head lice]], pubic ([[crab lice]]) and [[body lice]] | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions=[[eye irritation]], skin or [[scalp irritation]] | ||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult====== | |fdaLIADAdult=====Indications==== | ||
* treats [[head lice]], pubic (crab lice) and [[body lice]] | |||
* | ====Dosage==== | ||
* Important: Read warnings and full directions. For more information read the enclosed Consumer Information Leaflet. | |||
* For adults and children 6 months and over. | |||
* | =====APPLY===== | ||
* apply the entire contents of one tube thoroughly to dry hair, starting from behind the ears to the back of the neck, working forward. | |||
* | * to be effective, all lice and eggs must come in contact with product, ensure hair and scalp are saturated. For long or very thick hair two tubes may be required. | ||
=====COVER===== | |||
* | * use the shower cap supplied to keep hair damp, leave on for 4 hours before washing. | ||
* for serious infestation remove shower cap after 4 hours and leave product on overnight. | |||
=====WASH===== | |||
* wash area thoroughly with warm water and regular shampoo. Towel hair dry and comb out tangles using a regular comb or brush. | |||
===== | * as an option remove any remaining lice or nits with the included nit comb or fingernails. | ||
* if hair dries during combing dampen slightly with water. | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed===== | |fdaLIADPed=====Dosage==== | ||
* Important: Read warnings and full directions. For more information read the enclosed Consumer Information Leaflet. | |||
* | * For adults and children 6 months and over. | ||
=====APPLY===== | |||
* apply the entire contents of one tube thoroughly to dry hair, starting from behind the ears to the back of the neck, working forward. | |||
===== | * to be effective, all lice and eggs must come in contact with product, ensure hair and scalp are saturated. For long or very thick hair two tubes may be required. | ||
=====COVER===== | |||
* use the shower cap supplied to keep hair damp, leave on for 4 hours before washing. | |||
* for serious infestation remove shower cap after 4 hours and leave product on overnight. | |||
=====WASH===== | |||
* wash area thoroughly with warm water and regular shampoo. Towel hair dry and comb out tangles using a regular comb or brush. | |||
* as an option remove any remaining lice or nits with the included nit comb or fingernails. | |||
* if hair dries during combing dampen slightly with water. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=<!--Warnings--> | ||
|warnings=* For external use only. | |||
=====DO NOT USE===== | |||
* near eyes | |||
* inside the nose, mouth or vagina | |||
* on lice in eyebrows or eyelashes | |||
* See a doctor if lice are present in these areas. | |||
=====WHEN USING THIS PRODUCT===== | |||
* keep tightly closed and protect eyes with a washcloth or towel. | |||
* if product gets into the eyes, flush with water right away. | |||
=====STOP USE AND ASK A DOCTOR IF===== | |||
* eye irritation occurs | |||
* skin or scalp irritation continues or infection occurs | |||
* | =====KEEP OUT OF REACH OF CHILDREN===== | ||
* If swallowed, get medical help or contact a Poison Control Center right away. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials= | |clinicalTrials=* [[eye irritation]] | ||
* skin or [[scalp irritation]] | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions=<!--Use in Specific Populations--> | ||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA=* '''Pregnancy Category''' | |useInPregnancyFDA=* '''Pregnancy Category''' | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 265: | Line 114: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* Topical application | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 276: | Line 123: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=There is limited information regarding <i>Overdose</i> of {{PAGENAME}} in the drug label. | ||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox=: [[File:Quassia Wiki Str.png|thumb|none|600px|This image is provided by the Wikipedia.]] | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
<!--Structure--> | |||
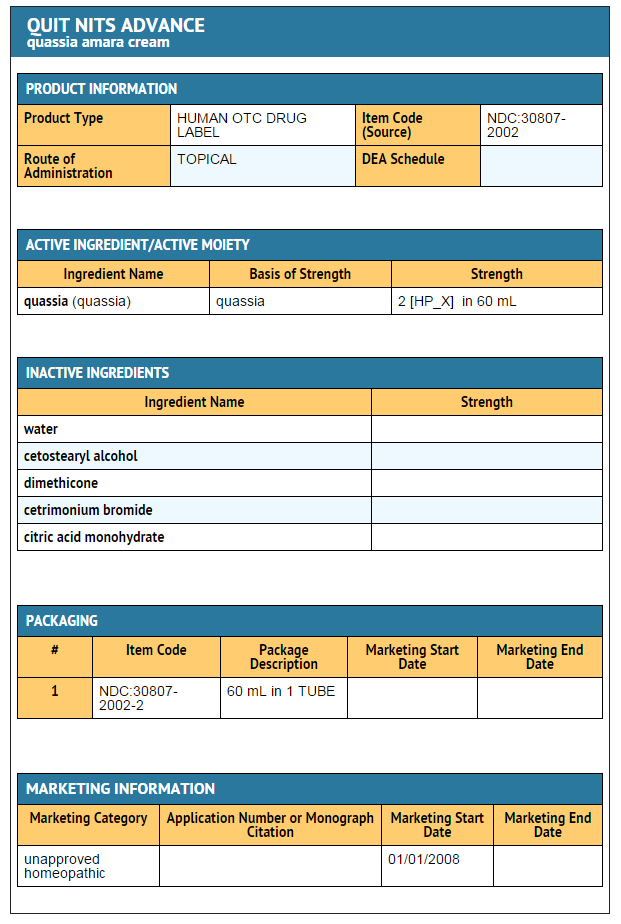
|structure=* ACTIVE INGREDIENTS | |||
:* Quassia amara 2X HPUS (Amargo 200mcg/g) | |||
* INACTIVE INGREDIENTS | |||
:* aqua, cetearyl alcohol, dimethicone, fragrance, cetrimonium bromide, citric acid | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 314: | Line 158: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied= | ||
|packLabel= | |storage=* keep carton for important product information | ||
* protect from excessive heat | |||
|packLabel=====PACKAGING INFORMATION==== | |||
Quit Nits® | |||
Caring for your child, naturally | |||
2.03 FL OZ (60mL) | |||
ADVANCE | |||
Kills head lice and eggs with one application | |||
WILD CHILD® | |||
Born, not made. | |||
Ingredients Incl: Aqua, cetearyl alcohol, dimethicone, fragrance, cetrimonium bromide, citric acid, quassia wood jamaican extract. | |||
4 simple steps to help eliminate Head Lice Infestation: | |||
<!-- | APPLY - Massage cream through dry hair and onto scalp, ensuring all of the hair and the back of the neck is wet with product. | ||
COVER - Use shower cap supplied to keep hair damp and leave on for 4 hours. For serious infestation remove cap and leave treatment on overnight. | |||
WASH - after 4 hours use your regular shampoo to remove the product. Any remaining lice and eggs should be removed with fingernails. | |||
FOLLOW-UP try Quit Nits Every Day Lice Preventative Spray to limit re-infestation. | |||
For external use only. Avoid contact with eyes. If product gets into eyes, rinse well with water. If irritation occurs, discontinue use. | |||
Manufactured for: | |||
Wild Child (US) Pty Ltd | |||
by The Triad Group | |||
Hartland, WI 53029 | |||
info@wildchildonline.com | |||
800.961.4936 | |||
For batch and expiry date see crimp | |||
: [[File:Quassia PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File:Quassia Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=800-961-4936 or | |||
support@QuitNits.com | |||
www.QuitNits.com | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* QUIT NITS ADVANCE®<ref>{{Cite web | title = Quassia amara | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=36682}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 17:02, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Quassia is a OTC cream that is FDA approved for the treatment of head lice, pubic (crab lice) and body lice. Common adverse reactions include eye irritation, skin or scalp irritation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Dosage
- Important: Read warnings and full directions. For more information read the enclosed Consumer Information Leaflet.
- For adults and children 6 months and over.
APPLY
- apply the entire contents of one tube thoroughly to dry hair, starting from behind the ears to the back of the neck, working forward.
- to be effective, all lice and eggs must come in contact with product, ensure hair and scalp are saturated. For long or very thick hair two tubes may be required.
COVER
- use the shower cap supplied to keep hair damp, leave on for 4 hours before washing.
- for serious infestation remove shower cap after 4 hours and leave product on overnight.
WASH
- wash area thoroughly with warm water and regular shampoo. Towel hair dry and comb out tangles using a regular comb or brush.
- as an option remove any remaining lice or nits with the included nit comb or fingernails.
- if hair dries during combing dampen slightly with water.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Quassia in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Quassia in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage
- Important: Read warnings and full directions. For more information read the enclosed Consumer Information Leaflet.
- For adults and children 6 months and over.
APPLY
- apply the entire contents of one tube thoroughly to dry hair, starting from behind the ears to the back of the neck, working forward.
- to be effective, all lice and eggs must come in contact with product, ensure hair and scalp are saturated. For long or very thick hair two tubes may be required.
COVER
- use the shower cap supplied to keep hair damp, leave on for 4 hours before washing.
- for serious infestation remove shower cap after 4 hours and leave product on overnight.
WASH
- wash area thoroughly with warm water and regular shampoo. Towel hair dry and comb out tangles using a regular comb or brush.
- as an option remove any remaining lice or nits with the included nit comb or fingernails.
- if hair dries during combing dampen slightly with water.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Quassia in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Quassia in pediatric patients.
Contraindications
There is limited information regarding Quassia Contraindications in the drug label.
Warnings
- For external use only.
DO NOT USE
- near eyes
- inside the nose, mouth or vagina
- on lice in eyebrows or eyelashes
- See a doctor if lice are present in these areas.
WHEN USING THIS PRODUCT
- keep tightly closed and protect eyes with a washcloth or towel.
- if product gets into the eyes, flush with water right away.
STOP USE AND ASK A DOCTOR IF
- eye irritation occurs
- skin or scalp irritation continues or infection occurs
KEEP OUT OF REACH OF CHILDREN
- If swallowed, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
- eye irritation
- skin or scalp irritation
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Quassia in the drug label.
Drug Interactions
There is limited information regarding Quassia Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Quassia in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Quassia during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Quassia with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Quassia with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Quassia with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Quassia with respect to specific gender populations.
Race
There is no FDA guidance on the use of Quassia with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Quassia in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Quassia in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Quassia in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Quassia in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical application
Monitoring
There is limited information regarding Monitoring of Quassia in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Quassia in the drug label.
Overdosage
There is limited information regarding Overdose of Quassia in the drug label.
Pharmacology
Mechanism of Action
There is limited information regarding Quassia Mechanism of Action in the drug label.
Structure
- ACTIVE INGREDIENTS
- Quassia amara 2X HPUS (Amargo 200mcg/g)
- INACTIVE INGREDIENTS
- aqua, cetearyl alcohol, dimethicone, fragrance, cetrimonium bromide, citric acid
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Quassia in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Quassia in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Quassia in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Quassia in the drug label.
How Supplied
There is limited information regarding Quassia How Supplied in the drug label.
Storage
- keep carton for important product information
- protect from excessive heat
Images
Drug Images
{{#ask: Page Name::Quassia |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PACKAGING INFORMATION
Quit Nits® Caring for your child, naturally
2.03 FL OZ (60mL)
ADVANCE Kills head lice and eggs with one application
WILD CHILD® Born, not made.
Ingredients Incl: Aqua, cetearyl alcohol, dimethicone, fragrance, cetrimonium bromide, citric acid, quassia wood jamaican extract. 4 simple steps to help eliminate Head Lice Infestation:
APPLY - Massage cream through dry hair and onto scalp, ensuring all of the hair and the back of the neck is wet with product.
COVER - Use shower cap supplied to keep hair damp and leave on for 4 hours. For serious infestation remove cap and leave treatment on overnight.
WASH - after 4 hours use your regular shampoo to remove the product. Any remaining lice and eggs should be removed with fingernails.
FOLLOW-UP try Quit Nits Every Day Lice Preventative Spray to limit re-infestation.
For external use only. Avoid contact with eyes. If product gets into eyes, rinse well with water. If irritation occurs, discontinue use.
Manufactured for: Wild Child (US) Pty Ltd by The Triad Group Hartland, WI 53029 info@wildchildonline.com 800.961.4936
For batch and expiry date see crimp
Ingredients and Appearance
{{#ask: Label Page::Quassia |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
800-961-4936 or
support@QuitNits.com
www.QuitNits.com
Precautions with Alcohol
- Alcohol-Quassia interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- QUIT NITS ADVANCE®[1]
Look-Alike Drug Names
There is limited information regarding Quassia Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.