Pegaptanib: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Pegaptanib": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (2 intermediate revisions by one other user not shown) | |||
| Line 6: | Line 6: | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=neovascular (wet) [[age-related macular degeneration]] | |indication=neovascular (wet) [[age-related macular degeneration]] | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions= | ||
[[anterior chamber inflammation]], [[blurred vision]], [[cataract]], [[conjunctival hemorrhage]], [[corneal edema]], [[eye discharge]], [[eye irritation]], [[eye pain]], [[hypertension]], [[increased intraocular pressure]] ([[IOP]]), [[ocular discomfort]], [[punctate keratitis]], reduced [[visual acuity]], [[visual disturbance]], [[vitreous floaters]], and [[vitreous opacities]]. | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 20: | Line 25: | ||
====Dosage==== | ====Dosage==== | ||
=====General Dosing Information===== | =====General Dosing Information===== | ||
* FOR OPHTHALMIC INTRAVITREAL INJECTION ONLY. | * FOR OPHTHALMIC [[INTRAVITREAL]] INJECTION ONLY. | ||
=====Dosing===== | =====Dosing===== | ||
* Macugen 0.3 mg should be administered once every six weeks by intravitreous injection into the eye to be treated. | * Macugen 0.3 mg should be administered once every six weeks by [[intravitreous]] injection into the eye to be treated. | ||
=====Preparation for Administration===== | =====Preparation for Administration===== | ||
| Line 70: | Line 75: | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings======Endophthalmitis===== | |warnings======Endophthalmitis===== | ||
* Intravitreous injections, including those with Macugen, have been associated with endophthalmitis. Proper aseptic injection technique should always be utilized when administering Macugen. In addition, patients should be monitored during the week following the injection to permit early treatment, should an infection occur. | * Intravitreous injections, including those with Macugen, have been associated with [[endophthalmitis]]. Proper aseptic injection technique should always be utilized when administering Macugen. In addition, patients should be monitored during the week following the injection to permit early treatment, should an infection occur. | ||
=====Increases in Intraocular Pressure===== | =====Increases in Intraocular Pressure===== | ||
| Line 85: | Line 90: | ||
=====Clinical Studies Experience===== | =====Clinical Studies Experience===== | ||
* The most frequently reported adverse events in patients treated with Macugen 0.3 mg for up to two years were [[anterior chamber inflammation]], [[blurred vision]], [[cataract]], [[conjunctival hemorrhage]], [[corneal edema]], [[eye discharge]], [[eye irritation]], [[eye pain]], [[hypertension]], increased intraocular pressure (IOP), [[ocular discomfort]], [[punctate keratitis]], reduced visual acuity, [[visual disturbance]], [[vitreous floaters]], and [[vitreous opacities]]. These events occurred in approximately 10-40% of patients. | * The most frequently reported adverse events in patients treated with Macugen 0.3 mg for up to two years were [[anterior chamber inflammation]], [[blurred vision]], [[cataract]], [[conjunctival hemorrhage]], [[corneal edema]], [[eye discharge]], [[eye irritation]], [[eye pain]], [[hypertension]], increased intraocular pressure (IOP), [[ocular discomfort]], [[punctate keratitis]], reduced [[visual acuity]], [[visual disturbance]], [[vitreous floaters]], and [[vitreous opacities]]. These events occurred in approximately 10-40% of patients. | ||
* The following events were reported in 6-10% of patients receiving Macugen 0.3 mg therapy: | * The following events were reported in 6-10% of patients receiving Macugen 0.3 mg therapy: | ||
| Line 127: | Line 132: | ||
* The injection procedure should be carried out under controlled aseptic conditions, which includes the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection. | * The injection procedure should be carried out under controlled aseptic conditions, which includes the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection. | ||
* The patient's medical history for hypersensitivity reactions should be evaluated prior to performing the intravitreal procedure. | * The patient's medical history for [[hypersensitivity reactions]] should be evaluated prior to performing the [[intravitreal]] procedure. | ||
* Following the injection, patients should be monitored for elevation in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and monitoring during the week following the injection. Patients should be instructed to report any symptoms suggestive of endophthalmitis without delay. | * Following the injection, patients should be monitored for elevation in [[intraocular pressure]] and for [[endophthalmitis]]. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, [[tonometry]] within 30 minutes following the injection, and monitoring during the week following the injection. Patients should be instructed to report any symptoms suggestive of [[endophthalmitis]] without delay. | ||
* No special dosage modification is required for any of the populations that have been studied (i.e. gender, elderly). | * No special dosage modification is required for any of the populations that have been studied (i.e. gender, elderly). | ||
| Line 186: | Line 191: | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=* Pegaptanib is a selective vascular endothelial growth factor (VEGF) antagonist. VEGF is a secreted protein that selectively binds and activates its receptors located primarily on the surface of vascular endothelial cells. VEGF induces angiogenesis, and increases vascular permeability and inflammation, all of which are thought to contribute to the progression of the neovascular (wet) form of age-related macular degeneration (AMD), a leading cause of blindness. VEGF has been implicated in blood retinal barrier breakdown and pathological ocular neovascularization. | |mechAction=* Pegaptanib is a selective [[vascular endothelial growth factor]] ([[VEGF]]) antagonist. VEGF is a secreted protein that selectively binds and activates its receptors located primarily on the surface of vascular endothelial cells. VEGF induces [[angiogenesis]], and increases vascular permeability and inflammation, all of which are thought to contribute to the progression of the neovascular (wet) form of age-related macular degeneration (AMD), a leading cause of blindness. VEGF has been implicated in blood retinal barrier breakdown and pathological ocular [[neovascularization]]. | ||
* Pegaptanib is an aptamer, a pegylated modified oligonucleotide, which adopts a three-dimensional conformation that enables it to bind to extracellular VEGF. Under in vitro testing conditions, pegaptanib binds to the major pathological VEGF isoform, extracellular VEGF165, thereby inhibiting VEGF165 binding to its VEGF receptors. The inhibition of VEGF164, the rodent counterpart of human VEGF165, was effective at suppressing pathological neovascularization. | * Pegaptanib is an aptamer, a pegylated modified oligonucleotide, which adopts a three-dimensional conformation that enables it to bind to extracellular VEGF. Under in vitro testing conditions, pegaptanib binds to the major pathological VEGF isoform, extracellular [[VEGF165]], thereby inhibiting VEGF165 binding to its VEGF receptors. The inhibition of VEGF164, the rodent counterpart of human VEGF165, was effective at suppressing pathological [[neovascularization]]. | ||
<!--Structure--> | <!--Structure--> | ||
| Line 211: | Line 216: | ||
|PK======Absorption===== | |PK======Absorption===== | ||
* In animals, pegaptanib is slowly absorbed into the systemic circulation from the eye after intravitreous administration. The rate of absorption from the eye is the rate limiting step in the disposition of pegaptanib in animals and is likely to be the rate limiting step in humans. | * In animals, pegaptanib is slowly absorbed into the systemic circulation from the eye after [[intravitreous]] administration. The rate of absorption from the eye is the rate limiting step in the disposition of pegaptanib in animals and is likely to be the rate limiting step in humans. | ||
* In humans, a mean maximum plasma concentration of about 80 ng/mL occurs within 1 to 4 days after a 3 mg monocular dose (10 times the recommended dose). The mean area under the plasma concentration-time curve (AUC) is about 25 μg•hr/mL at this dose. | * In humans, a mean maximum plasma concentration of about 80 ng/mL occurs within 1 to 4 days after a 3 mg monocular dose (10 times the recommended dose). The mean area under the plasma concentration-time curve (AUC) is about 25 μg•hr/mL at this dose. | ||
* Pegaptanib is metabolized by nucleases and is generally not affected by the cytochrome P450 system. | * Pegaptanib is metabolized by nucleases and is generally not affected by the [[cytochrome P450]] system. | ||
* Two early clinical studies conducted in patients who received Macugen alone and in combination with photodynamic therapy (PDT) revealed no apparent difference in the plasma pharmacokinetics of pegaptanib. | * Two early clinical studies conducted in patients who received Macugen alone and in combination with photodynamic therapy (PDT) revealed no apparent difference in the plasma pharmacokinetics of pegaptanib. | ||
| Line 221: | Line 226: | ||
=====Distribution/Metabolism/Excretion===== | =====Distribution/Metabolism/Excretion===== | ||
* Twenty-four hours after intravitreous administration of a radiolabeled dose of pegaptanib to both eyes of rabbits, radioactivity was mainly distributed in vitreous fluid, retina, and aqueous fluid. After intravitreous and intravenous administrations of radiolabeled pegaptanib to rabbits, the highest concentrations of radioactivity (excluding the eye for the intravitreous dose) were obtained in the kidney. In rabbits, the component nucleotide, 2'-fluorouridine is found in plasma and urine after single radiolabeled pegaptanib intravenous and intravitreous doses. In rabbits, pegaptanib is eliminated as parent drug and metabolites primarily in the urine. | * Twenty-four hours after intravitreous administration of a radiolabeled dose of pegaptanib to both eyes of rabbits, radioactivity was mainly distributed in vitreous fluid, retina, and aqueous fluid. After [[intravitreous]] and intravenous administrations of radiolabeled pegaptanib to rabbits, the highest concentrations of radioactivity (excluding the eye for the intravitreous dose) were obtained in the kidney. In rabbits, the component nucleotide, 2'-fluorouridine is found in plasma and urine after single radiolabeled pegaptanib intravenous and [[intravitreous]] doses. In rabbits, pegaptanib is eliminated as parent drug and metabolites primarily in the urine. | ||
* Based on preclinical data, pegaptanib is metabolized by endo- and exonucleases. | * Based on preclinical data, pegaptanib is metabolized by endo- and exonucleases. | ||
| Line 236: | Line 241: | ||
=====Animal Toxicology and/or Pharmacology===== | =====Animal Toxicology and/or Pharmacology===== | ||
* Pegaptanib and its monomer component nucleotides (2'-MA, 2'-MG, 2'-FU, 2'-FC) were evaluated for genotoxicity in a battery of in vitro and in vivo assay systems. Pegaptanib, 2'-O-methyladenosine (2'-MA), and 2'-O-methylguanosine (2'-MG) were negative in all assay systems evaluated. 2'-fluorouridine (2'-FU) and 2'-fluorocytidine (2'-FC) were nonclastogenic and were negative in all S. typhimurium tester strains, but produced a non-dose related increase in revertant frequency in a single E. coli tester strain. Pegaptanib, 2'-FU, and 2'-FC tested negative in cell transformation assays. | * Pegaptanib and its monomer component nucleotides (2'-MA, 2'-MG, 2'-FU, 2'-FC) were evaluated for genotoxicity in a battery of in vitro and in vivo assay systems. Pegaptanib, 2'-O-methyladenosine (2'-MA), and 2'-O-methylguanosine (2'-MG) were negative in all assay systems evaluated. 2'-fluorouridine (2'-FU) and 2'-fluorocytidine (2'-FC) were nonclastogenic and were negative in all [[S. typhimurium]] tester strains, but produced a non-dose related increase in revertant frequency in a single E. coli tester strain. Pegaptanib, 2'-FU, and 2'-FC tested negative in cell transformation assays. | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=* Macugen was studied in two controlled, double-masked, and identically designed randomized studies in patients with neovascular AMD. Patients were randomized to receive control (sham treatment) or 0.3 mg, 1 mg or 3 mg Macugen administered as intravitreous injections every 6 weeks for 48 weeks. A total of approximately 1200 patients were enrolled with 892 patients receiving Macugen and 298 receiving a sham injection. The median age of the patients was 77 years. Patients received a mean 8.5 treatments out of a possible 9 total treatments across all treatment arms. Patients were re-randomized between treatment and no treatment during the second year. Patients who continued treatment in year 2 received a mean of 16 treatments out of a possible total 17 overall. | |clinicalStudies=* Macugen was studied in two controlled, double-masked, and identically designed randomized studies in patients with neovascular AMD. Patients were randomized to receive control (sham treatment) or 0.3 mg, 1 mg or 3 mg Macugen administered as [[intravitreous]] injections every 6 weeks for 48 weeks. A total of approximately 1200 patients were enrolled with 892 patients receiving Macugen and 298 receiving a sham injection. The median age of the patients was 77 years. Patients received a mean 8.5 treatments out of a possible 9 total treatments across all treatment arms. Patients were re-randomized between treatment and no treatment during the second year. Patients who continued treatment in year 2 received a mean of 16 treatments out of a possible total 17 overall. | ||
* The two trials enrolled patients with neovascular AMD characteristics including classic, occult, and mixed lesions of up to 12 disc areas and baseline visual acuity in the study eye between 20/40 and 20/320. The primary efficacy endpoint was the proportion of patients losing less than 15 letters of visual acuity, from baseline up to 54 week assessment. Verteporfin PDT usage was permitted at the discretion of the investigators in patients with predominantly classic lesions. | * The two trials enrolled patients with neovascular AMD characteristics including classic, occult, and mixed lesions of up to 12 disc areas and baseline visual acuity in the study eye between 20/40 and 20/320. The primary efficacy endpoint was the proportion of patients losing less than 15 letters of visual acuity, from baseline up to 54 week assessment. Verteporfin PDT usage was permitted at the discretion of the investigators in patients with predominantly classic lesions. | ||
| Line 292: | Line 297: | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 16:53, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Pegaptanib is a Ophthalmologic Agent that is FDA approved for the treatment of neovascular (wet) age-related macular degeneration. Common adverse reactions include anterior chamber inflammation, blurred vision, cataract, conjunctival hemorrhage, corneal edema, eye discharge, eye irritation, eye pain, hypertension, increased intraocular pressure (IOP), ocular discomfort, punctate keratitis, reduced visual acuity, visual disturbance, vitreous floaters, and vitreous opacities..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Macugen is indicated for the treatment of neovascular (wet) age-related macular degeneration.
Dosage
General Dosing Information
- FOR OPHTHALMIC INTRAVITREAL INJECTION ONLY.
Dosing
- Macugen 0.3 mg should be administered once every six weeks by intravitreous injection into the eye to be treated.
Preparation for Administration
- Macugen should be inspected visually for particulate matter and discoloration prior to administration. If visible particulates are observed and/or the liquid in the syringe is discolored, the syringe must not be used.
- Administration of the syringe contents involves assembly of the syringe with the administration needle. The injection procedure should be carried out under controlled aseptic conditions, which includes the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). When ready to assemble syringe and administer injection, carefully peel open pouches, remove contents, and place on sterile field. If upon opening the pouch, the plastic clip is missing or not attached to the syringe, the syringe should not be used.
- To avoid compromising the sterility of the product, do not pull back on the plunger.
- Remove the syringe from the plastic clip.
- Twist off cap.
- Attach the sterile BD® 30G 1/2" Precision Glide® administration needle (included) to the syringe by screwing it into the syringe tip.
--Another sterile BD® 30G 1/2" Precision Glide® administration needle may be used in lieu of the one included. Remove the plastic needle shield from the needle. Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top of the syringe. SLOWLY depress the plunger to eliminate all the bubbles and to expel the excess drug so that the top edge of the 3rd rib on the plunger stopper aligns with the pre-printed black dosing line (See Figure 2, below). Inject the entire contents of the syringe.
DOSAGE FORMS AND STRENGTHS
- Single-use glass syringe pre-filled with 0.3 mg of Macugen® in a nominal 90 μL solution for intravitreal injection.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pegaptanib in adult patients.
Non–Guideline-Supported Use
- Diabetic macular edema[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Pegaptanib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pegaptanib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pegaptanib in pediatric patients.
Contraindications
Ocular or Periocular Infections
- Macugen is contraindicated in patients with ocular or periocular infections.
Hypersensitivity
- Macugen is contraindicated in patients with known hypersensitivity to pegaptanib sodium or any other excipient in this product.
Warnings
Endophthalmitis
- Intravitreous injections, including those with Macugen, have been associated with endophthalmitis. Proper aseptic injection technique should always be utilized when administering Macugen. In addition, patients should be monitored during the week following the injection to permit early treatment, should an infection occur.
Increases in Intraocular Pressure
- Increases in intraocular pressure have been seen within 30 minutes of injection with Macugen. Therefore, intraocular pressure as well as the perfusion of the optic nerve head should be monitored and managed appropriately.
Anaphylaxis
- Rare cases of anaphylaxis/anaphylactoid reactions, including angioedema, have been reported in the post-marketing experience following the Macugen intravitreal administration procedure
Adverse Reactions
Clinical Trials Experience
Injection Procedure
- Serious adverse events related to the injection procedure occurring in < 1% of intravitreous injections included endophthalmitis, retinal detachment, and iatrogenic traumatic cataract.
Clinical Studies Experience
- The most frequently reported adverse events in patients treated with Macugen 0.3 mg for up to two years were anterior chamber inflammation, blurred vision, cataract, conjunctival hemorrhage, corneal edema, eye discharge, eye irritation, eye pain, hypertension, increased intraocular pressure (IOP), ocular discomfort, punctate keratitis, reduced visual acuity, visual disturbance, vitreous floaters, and vitreous opacities. These events occurred in approximately 10-40% of patients.
- The following events were reported in 6-10% of patients receiving Macugen 0.3 mg therapy:
Ocular
Non-Ocular
- The following events were reported in 1-5% of patients receiving Macugen 0.3 mg therapy:
Ocular
- allergic conjunctivitis, conjunctival edema, corneal abrasion, corneal deposits, corneal epithelium disorder, endophthalmitis, eye inflammation, eye swelling, eyelid irritation, meibomianitis, mydriasis, periorbital hematoma, retinal edema, vitreous hemorrhage.
Non-Ocular
- arthritis, bone spur, carotid artery occlusion, cerebrovascular accident, chest pain, contact dermatitis, contusion, diabetes mellitus, dyspepsia, hearing loss, pleural effusion, transient ischemic attack, urinary retention, vertigo, vomiting.
Postmarketing Experience
- Anaphylaxis/anaphylactoid reactions, including angioedema, have been identified during postapproval use of Macugen. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure
Drug Interactions
Use in Specific Populations
Pregnancy
- Pegaptanib produced no maternal toxicity and no evidence of teratogenicity or fetal mortality in mice at intravenous doses of up to 40 mg/kg/day (about 7,000 times the recommended human monocular ophthalmic dose of 0.3 mg/eye). Pegaptanib crosses the placenta in mice.
- There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pegaptanib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pegaptanib during labor and delivery.
Nursing Mothers
- It is not known whether pegaptanib is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Macugen is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness of Macugen in pediatric patients have not been established.
Geriatic Use
- Approximately 94% (834/892) of the patients treated with Macugen were ≥ 65 years of age and approximately 62% (553/892) were ≥ 75 years of age. No difference in treatment effect or systemic exposure was seen with increasing age.
Gender
There is no FDA guidance on the use of Pegaptanib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pegaptanib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pegaptanib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pegaptanib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pegaptanib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pegaptanib in patients who are immunocompromised.
Administration and Monitoring
Administration
- FOR OPHTHALMIC INTRAVITREAL INJECTION ONLY
- The injection procedure should be carried out under controlled aseptic conditions, which includes the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
- The patient's medical history for hypersensitivity reactions should be evaluated prior to performing the intravitreal procedure.
- Following the injection, patients should be monitored for elevation in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and monitoring during the week following the injection. Patients should be instructed to report any symptoms suggestive of endophthalmitis without delay.
- No special dosage modification is required for any of the populations that have been studied (i.e. gender, elderly).
- The safety and efficacy of Macugen therapy administered to both eyes concurrently have not been studied.
Monitoring
There is limited information regarding Monitoring of Pegaptanib in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Pegaptanib in the drug label.
Overdosage
- Doses of Macugen up to 10 times the recommended dosage of 0.3 mg have been studied. No additional adverse events have been noted but there is decreased efficacy with doses above 1 mg.
Pharmacology

| |
Pegaptanib sodium
| |
| Systematic (IUPAC) name | |
| RNA, ((2'-deoxy-2'-fluoro)C-Gm-Gm-A-A-(2'-deoxy-2'-fluoro)U-(2'-deoxy-2'-fluoro)C-Am-Gm-(2'-deoxy-2'-fluoro)U-Gm-Am-Am-(2'-deoxy-2'-fluoro)U-Gm-(2'-deoxy-2'-fluoro)C-(2'-deoxy-2'-fluoro)U-(2'-deoxy-2'fluoro)U-Am-(2'-deoxy-2'-fluoro)U-Am-(2'-deoxy-2'-fluoro)C-Am-(2'-deoxy-2'-fluoro)U-(2'deoxy-2'-fluoro)C-(2'-deoxy-2'-fluoro)C-Gm-(3'→3')-dT), 5'-ester with α,α'-[4,12-dioxo-6[[[5-(phosphoonoxy)pentyl]amino]carbonyl]-3,13-dioxa-5,11-diaza-1,15-pentadecanediyl]bis[ω-methoxypoly(oxy-1,2-ethanediyl)], sodium salt[2] | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | C294H342F13N107Na28O188P28[C2H4O]2n (n≈900) |
| Mol. mass | ~50 kDa |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 10 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravitreal injection |
Mechanism of Action
- Pegaptanib is a selective vascular endothelial growth factor (VEGF) antagonist. VEGF is a secreted protein that selectively binds and activates its receptors located primarily on the surface of vascular endothelial cells. VEGF induces angiogenesis, and increases vascular permeability and inflammation, all of which are thought to contribute to the progression of the neovascular (wet) form of age-related macular degeneration (AMD), a leading cause of blindness. VEGF has been implicated in blood retinal barrier breakdown and pathological ocular neovascularization.
- Pegaptanib is an aptamer, a pegylated modified oligonucleotide, which adopts a three-dimensional conformation that enables it to bind to extracellular VEGF. Under in vitro testing conditions, pegaptanib binds to the major pathological VEGF isoform, extracellular VEGF165, thereby inhibiting VEGF165 binding to its VEGF receptors. The inhibition of VEGF164, the rodent counterpart of human VEGF165, was effective at suppressing pathological neovascularization.
Structure
- Macugen (pegaptanib sodium injection) is a sterile, aqueous solution containing pegaptanib sodium for intravitreous injection. Macugen is supplied in a single-dose, pre-filled syringe and is formulated as a 3.47 mg/mL solution, measured as the free acid form of the oligonucleotide. The active ingredient is 0.3 mg of the free acid form of the oligonucleotide without polyethylene glycol, in a nominal volume of 90 μL. This dose is equivalent to 1.6 mg of pegaptanib sodium (pegylated oligonucleotide) or 0.32 mg when expressed as the sodium salt form of the oligonucleotide moiety. The product is a sterile, clear, preservative-free solution containing sodium chloride, monobasic sodium phosphate monohydrate, dibasic sodium phosphate heptahydrate, hydrochloric acid, and/or sodium hydroxide to adjust the pH and water for injection.
- Pegaptanib sodium is a covalent conjugate of an oligonucleotide of twenty-eight nucleotides in length that terminates in a pentylamino linker, to which two 20-kilodalton monomethoxy polyethylene glycol (PEG) units are covalently attached via the two amino groups on a lysine residue.
- Pegaptanib sodium is represented by the following structural formula:
- The chemical name for pegaptanib sodium is as follows: RNA, ((2'-deoxy-2'-fluoro)C-Gm-Gm-A-A-(2'-deoxy-2'-fluoro)U-(2'-deoxy-2'-fluoro)C-Am-Gm-(2'-deoxy-2'-fluoro)U-Gm-Am-Am-(2'-deoxy-2'-fluoro)U-Gm-(2'-deoxy-2'-fluoro)C-(2'-deoxy-2'-fluoro)U-(2'-deoxy-2'-fluoro)U-Am-(2'-deoxy-2'-fluoro)U-Am-(2'-deoxy-2'-fluoro)C-Am-(2'-deoxy-2'-fluoro)U-(2'-deoxy-2'-fluoro)C-(2'-deoxy-2'-fluoro)C-Gm-(3'→3')-dT), 5'-ester with α,α'-[4,12-dioxo-6-5-(phosphoonoxy)pentylamino carbonyl]-3,13-dioxa-5,11-diaza-1,15-pentadecanediyl]bis[ω-methoxypoly(oxy-1,2-ethanediyl)], sodium salt.
- The molecular formula for pegaptanib sodium is C294H342F13N107Na28O188P28[C2H4O]n (where n is approximately 900) and the molecular weight is approximately 50 kilodaltons.
- Macugen is formulated to have an osmolality of 280-360 mOsm/Kg, and a pH of 6-7.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Pegaptanib in the drug label.
Pharmacokinetics
Absorption
- In animals, pegaptanib is slowly absorbed into the systemic circulation from the eye after intravitreous administration. The rate of absorption from the eye is the rate limiting step in the disposition of pegaptanib in animals and is likely to be the rate limiting step in humans.
- In humans, a mean maximum plasma concentration of about 80 ng/mL occurs within 1 to 4 days after a 3 mg monocular dose (10 times the recommended dose). The mean area under the plasma concentration-time curve (AUC) is about 25 μg•hr/mL at this dose.
- Pegaptanib is metabolized by nucleases and is generally not affected by the cytochrome P450 system.
- Two early clinical studies conducted in patients who received Macugen alone and in combination with photodynamic therapy (PDT) revealed no apparent difference in the plasma pharmacokinetics of pegaptanib.
Distribution/Metabolism/Excretion
- Twenty-four hours after intravitreous administration of a radiolabeled dose of pegaptanib to both eyes of rabbits, radioactivity was mainly distributed in vitreous fluid, retina, and aqueous fluid. After intravitreous and intravenous administrations of radiolabeled pegaptanib to rabbits, the highest concentrations of radioactivity (excluding the eye for the intravitreous dose) were obtained in the kidney. In rabbits, the component nucleotide, 2'-fluorouridine is found in plasma and urine after single radiolabeled pegaptanib intravenous and intravitreous doses. In rabbits, pegaptanib is eliminated as parent drug and metabolites primarily in the urine.
- Based on preclinical data, pegaptanib is metabolized by endo- and exonucleases.
- In humans, after a 3 mg monocular dose (10 times the recommended dose), the average (± standard deviation) apparent plasma half-life of pegaptanib is 10 (±4) days.
Special Populations
- Plasma concentrations do not appear to be affected by age or gender, but have not been studied in patients under the age of 50.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies with pegaptanib have not been conducted. No data are available to evaluate male or female mating or fertility indices.
Animal Toxicology and/or Pharmacology
- Pegaptanib and its monomer component nucleotides (2'-MA, 2'-MG, 2'-FU, 2'-FC) were evaluated for genotoxicity in a battery of in vitro and in vivo assay systems. Pegaptanib, 2'-O-methyladenosine (2'-MA), and 2'-O-methylguanosine (2'-MG) were negative in all assay systems evaluated. 2'-fluorouridine (2'-FU) and 2'-fluorocytidine (2'-FC) were nonclastogenic and were negative in all S. typhimurium tester strains, but produced a non-dose related increase in revertant frequency in a single E. coli tester strain. Pegaptanib, 2'-FU, and 2'-FC tested negative in cell transformation assays.
Clinical Studies
- Macugen was studied in two controlled, double-masked, and identically designed randomized studies in patients with neovascular AMD. Patients were randomized to receive control (sham treatment) or 0.3 mg, 1 mg or 3 mg Macugen administered as intravitreous injections every 6 weeks for 48 weeks. A total of approximately 1200 patients were enrolled with 892 patients receiving Macugen and 298 receiving a sham injection. The median age of the patients was 77 years. Patients received a mean 8.5 treatments out of a possible 9 total treatments across all treatment arms. Patients were re-randomized between treatment and no treatment during the second year. Patients who continued treatment in year 2 received a mean of 16 treatments out of a possible total 17 overall.
- The two trials enrolled patients with neovascular AMD characteristics including classic, occult, and mixed lesions of up to 12 disc areas and baseline visual acuity in the study eye between 20/40 and 20/320. The primary efficacy endpoint was the proportion of patients losing less than 15 letters of visual acuity, from baseline up to 54 week assessment. Verteporfin PDT usage was permitted at the discretion of the investigators in patients with predominantly classic lesions.
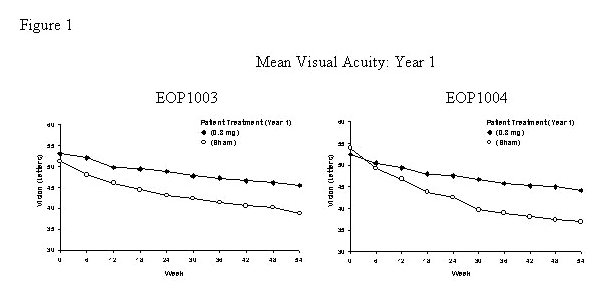
- The groups treated with Macugen 0.3 mg exhibited a statistically significant result in both trials for the primary efficacy endpoint at 1 year: Study EOP1003, Macugen 73% vs. Sham 60%; Study EOP1004, Macugen 67% vs. Sham 53%. Concomitant use of PDT overall was low. More sham treated patients (75/296) received PDT than Macugen 0.3 mg treated patients (58/294).
- On average, Macugen 0.3 mg treated patients and sham treated patients continued to experience vision loss. However, the rate of vision decline in the Macugen treated group was slower than the rate in the patients who received sham treatment. See Figure 1.
- At the end of the first year (week 54), approximately 1050 of the original 1200 patients were re-randomized to either continue the same treatment or to discontinue treatment through week 102. See Figure 2.
- Macugen was less effective during the second year than during the first year. The percentage of patients losing less than 15 letters from baseline to week 102 was: Study EOP1003, Macugen 38/67 (57%); Sham 30/54 (56%); Study EOP1004, Macugen 40/66 (61%); Sham 18/53 (34%).
- Dose levels above 0.3 mg did not demonstrate any additional benefit.
- The safety or efficacy of Macugen beyond 2 years has not been demonstrated.
How Supplied
- Macugen (pegaptanib sodium injection) is supplied in a sterile foil pouch as a single-use glass syringe pre-filled with 0.3 mg of Macugen® in a nominal 90 μL deliverable volume pack. A sterile packaged BD® single use 30G x 1/2" Precision Glide® Luer Lok® needle is supplied in a separate pouch. The foil pouch and needle are packaged together in a carton (NDC 68782-001-02).
Storage
- Store in the refrigerator at 2° to 8°C (36° to 46°F). Do not freeze or shake vigorously.
Images
Drug Images
{{#ask: Page Name::Pegaptanib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 68782-001-02
Rx only
Macugen® (pegaptanib sodium injection)
For Intravitreous Injection
0.3 mg / 90 μL*
Ingredients and Appearance
{{#ask: Label Page::Pegaptanib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- In the days following Macugen administration, patients are at risk for the development of endophthalmitis. If the eye becomes red, sensitive to light, painful or develops a change in vision, the patient should seek the immediate care with their ophthalmologist
Precautions with Alcohol
- Alcohol-Pegaptanib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- MACUGEN®[3]
Look-Alike Drug Names
There is limited information regarding Pegaptanib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Cunningham ET, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ; et al. (2005). "A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema". Ophthalmology. 112 (10): 1747–57. doi:10.1016/j.ophtha.2005.06.007. PMID 16154196.
- ↑ Drug Information: Pegaptanib Sodium Injection
- ↑ "Pegaptanib sodium".