Nitisinone: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
m (Protected "Nitisinone": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (9 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag=<!--Overview--> | |authorTag=<!--Overview-->{{RB}} | ||
|genericName=Nitisinone | |||
|aOrAn=a | |aOrAn=a | ||
| | |drugClass=Gastrointestinal Agent | ||
|adverseReactions=<!--Black Box Warning--> | |indicationType=treatment | ||
|indication=[[hereditary tyrosinemia type 1]] (HT-1) | |||
|adverseReactions=[[hepatic neoplasm]], [[liver failure]], [[thrombocytopenia]], [[leucopenia]], [[visual system complaints]] including [[conjunctivitis]], [[corneal opacity]], [[keratitis]], and [[photophobia]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult====== | |fdaLIADAdult=====Indications==== | ||
* Orfadin(R) capsules (nitisinone) are indicated as an adjunct to dietary restriction of [[tyrosine]] and [[phenylalanine]] in the treatment of [[hereditary tyrosinemia type 1]] | |||
* | |||
====Dosage==== | |||
* The recommended dose of Orfadin is 1 to 2 mg/kg divided into two daily doses. The initial dose is 1 mg/kg/day divided for morning and evening administration. Treatment with Orfadin should be initiated by a physician experienced in the treatment of [[HT-1]]. | |||
* The dose of Orfadin may be adjusted in each patient. In patients whose erythrocyte PBG-synthase activity and urine 5-ALA and urine [[succinylacetone]] are not normalized within one month after the start of Orfadin treatment, the Orfadin dose may be increased to 1.5 mg/kg/day. In patients receiving 1.5 mg/kg/day, whose erythrocyte PBG-synthase activity and urine 5-ALA and urine [[succinylacetone]] remain elevated and whose plasma [[succinylacetone]] is not normalized after three months, the dose may be increased to up a maximum dose of 2 mg/kg/day. | |||
* | * If plasma nitisinone concentration, plasma [[succinylacetone]], urine [[5-ALA]] and erythrocyte PBG-synthase activity are not available, clinical laboratory assessments should include urine [[succinyl acetone]], liver function tests, [[alpha fetoprotein]], and serum [[tyrosine]] and [[phenylalanine]] level. During initiation of therapy and during acute exacerbations, it may be necessary to follow more closely all available biochemical parameters | ||
====DOSAGE FORMS AND STRENGTHS==== | |||
* Orfadin capsules (nitisinone) are available as white capsules imprinted with ”NTBC” followed by "2 mg", "5 mg", or "10 mg", indicating the actual amount of nitisinone in each capsule. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
* | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed====== | |fdaLIADPed=====Indications==== | ||
* Orfadin(R) capsules (nitisinone) are indicated as an adjunct to dietary restriction of [[tyrosine]] and [[phenylalanine]] in the treatment of [[hereditary tyrosinemia type 1]] (HT-1) | |||
* | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ====Dosage==== | ||
* The recommended dose of Orfadin is 1 to 2 mg/kg divided into two daily doses. The initial dose is 1 mg/kg/day divided for morning and evening administration. Treatment with Orfadin should be initiated by a physician experienced in the treatment of HT-1. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=* None | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=* | |warnings======High Plasma Tyrosine Levels===== | ||
* Orfadin is an inhibitor of [[4-hydroxyphenyl-pyruvate dioxygenase]], an enzyme in the [[tyrosine]] metabolic pathway. Therefore, treatment with Orfadin may cause an increase in blood [[tyrosine]] in patients with HT-1, and patients should maintain concomitant reduction in dietary [[tyrosine]] and [[phenylalanine]] while on Orfadin treatment. Plasma tyrosine levels should be maintained below 500 μmol/L. Inadequate restriction of [[tyrosine]] and [[phenylalanine]] intake may increase blood [[tyrosine]] levels and may be associated with blood tyrosine levels greater than 500 μmol/L leading to the following: | |||
* Ocular signs and symptoms including [[corneal ulcers]], [[corneal opacities]], [[keratitis]], [[conjunctivitis]], [[eye pain]], and [[photophobia]] have been reported in patients treated with Orfadin. Therefore, ophthalmologic examination including slit-lamp examination should be performed prior to initiating Orfadin treatment. Patients who develop [[photophobia]], [[eye pain]], or signs of inflammation such as [[redness]], [[swelling]], or [[burning of the eyes]] during treatment with Orfadin should undergo slit-lamp reexamination and immediate measurement of the plasma tyrosine concentration. | |||
* Variable degrees of mental retardation and developmental delay. In patients treated with Orfadin who exhibit an abrupt change in neurologic status, a clinical laboratory assessment including plasma tyrosine levels should be performed. | |||
* Painful hyperkeratotic plaques on the soles and palms. | |||
* In patients with HT-1 treated with dietary restrictions and Orfadin who develop elevated plasma tyrosine levels, an assessment of dietary tyrosine intake should be performed. | |||
* | =====LEUCOPENIA AND SEVERE THROMBOCYTOPENIA===== | ||
* In clinical trials, patients treated with Orfadin and dietary restriction developed transient [[leucopenia]] (3%), [[thrombocytopenia]] (3%), or both (1.5%). One patient who developed both [[leucopenia]] and [[thrombocytopenia]] improved after the dose of Orfadin was decreased from 2 mg/kg to 1 mg/kg. No patients developed infections or bleeding as a result of the episodes of [[leucopenia]] and [[thromobocytopenia]]. [[Platelet]] and [[white blood cell counts]] should be monitored regularly during nitisinone therapy. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials= | |clinicalTrials=The following serious adverse reactions are described below and elsewhere in the labeling: | ||
[[High Plasma Tyrosine Levels]]. | |||
* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
====CLINICAL TRIALS EXPERIENCE==== | |||
* Orfadin was studied in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 21.7 years at enrollment (median age 9 months), who were diagnosed with [[HT-1]] by the presence of [[succinyl-acetone]] in the urine or plasma. The starting dose of Orfadin was 0.6 to 1 mg/kg/day, and the dose was increased in some patients to 2 mg/kg/day based on weight, biochemical, and enzyme markers. Median duration of treatment was 22.2 months (range 0.1 to 80 months). | |||
* The most serious adverse reactions reported during Orfadin treatment were [[thrombocytopenia]], [[leucopenia]], [[porphyria]], and ocular/visual complaints. Most patients with ocular/visual events had transient symptoms lasting less than one week, while 6 patients had symptoms lasting 16 to 672 days. Six patients had [[thrombocytopenia]], with platelet counts 30,000/uL or lower in 3 patients. In 4 patients with [[thrombocytopenia]], [[platelet counts]] returned to normal without change in Orfadin dose. In 2 patients platelet count returned to normal 2 weeks to 5 months after Orfadin treatment was discontinued. No patients developed infections or bleeding as a result of the episodes of [[leucopenia]] and [[thrombocytopenia]]. | |||
* Other serious adverse events reported during Orfadin treatment were [[hepatic neoplasm]], [[liver failure]], and [[porphyric crises]]. Patients with [[hereditary tyrosinemia type 1]] are at increased risk of developing [[porphyric crises]], [[hepatic neoplasms]], and [[liver failure]] requiring [[liver transplantation]]. These complications of HT-1 were observed in patients treated with nitisinone for a median of 22 months during the clinical trial ([[liver transplantation]] 13%, [[liver failure]] 7%, [[malignant hepatic neoplasms]] 5%, [[benign hepatic neoplasms]] 3%, [[porphyria]] 0.5%). Regular monitoring for these complications by hepatic imaging ([[ultrasound]], [[computerized tomography]], [[magnetic resonance imaging]]) and laboratory tests, including serum [[alpha-fetoprotein]] concentration is recommended. Patients with increasing [[alpha-fetoprotein]] levels or development of liver nodules during treatment with nitisinone should be evaluated for [[hepatic malignancy]]. | |||
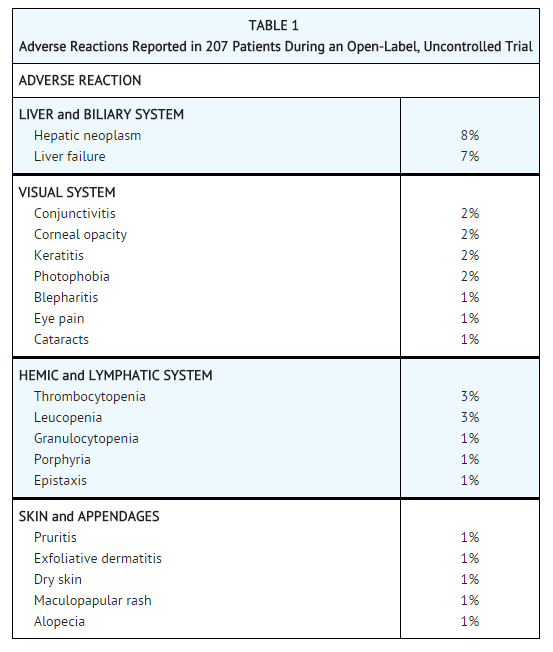
* The most common adverse reactions reported in the clinical trial are summarized in Table 1. | |||
: [[File:Nitisinone Adv Eff.png|none|500px]] | |||
* Adverse reactions reported in less than 1% of the patients, regardless of causality assessment, included death, [[seizure]], [[brain tumor]], [[encephalopathy]], [[headache]], [[hyperkinesia]], [[cyanosis]], [[abdominal pain]], [[diarrhea]], [[enanthema]], [[gastritis]], [[gastroenteritis]], [[gastrointestinal hemorrhage]], [[melena]], [[tooth discoloration]], [[hepatic function]] disorder, elevated [[hepatic enzymes]], [[liver enlargement]], [[dehydration]], [[hypoglycemia]], [[thirst]], [[infection]], [[septicemia]], [[otitis]], infection (not otherwise specified), [[bronchitis]], [[respiratory insufficiency]], [[pathologic fracture]], [[amenorrhea]], [[nervousness]], and [[somnolence]]. | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
<!--Use in Specific Populations--> | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=* Reproduction studies have been performed in mice at oral doses of about 0.4, 4 and 20 times the recommended human dose (1 mg/kg/day) and in rabbits at oral doses of about 1.6, 4 and 8 times the recommended human dose based on the body surface area. In mice, nitisinone has been shown to cause incomplete skeletal ossification of fetal bones at 0.4, 4 and 20 times the recommended human dose, increased gestational length at 4 and 20 times the recommended human dose, and decreased pup survival at 0.4 times the recommended human dose based on the body surface area. In rabbits, nitisinone caused incomplete skeletal ossification of fetal bones at 1.6, 4 and 8 times the recommended human dose based on the body surface area. | |||
* However, there are no adequate and well controlled studies in pregnant women. Nitisinone should be used during pregnancy only if the potential benefit justified the potential risk to the fetus. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
===== | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* Although the exposure was not quantified naive pups that were exposed to Orfadin via breast milk showed signs of ocular toxicity and lower body weight. This suggests that Orfadin is excreted via breast milk in rats. It is not known whether Orfadin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Orfadin is administered to a nursing woman. | |||
|useInPed=* Pediatric patients with HT-1, ages birth to 17 years have been treated with Orfadin in one open-label, uncontrolled clinical study. Monitoring of blood and urine succinyl acetone levels are recommended in the children to ensure adequate control. A nutritionist skilled in managing children with inborn errors of metabolism should be employed to design a low-protein diet deficient in tyrosine and phenylalanine. | |||
|useInGeri=* Clinical studies of nitisinone did not include any subjects aged 65 and over to determine whether they respond differently from younger subjects. HT-1 is presently a disease of the pediatric population. No pharmacokinetic studies of nitisinone have been performed in geriatric patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy in this patient population. | |||
|useInGender=* The effect of gender on the pharmacokinetics of Orfadin has not been studied. | |||
|useInRace=* The effect of race on the pharmacokinetics of Orfadin has not been studied. | |||
|useInRenalImpair=* The effect of renal insufficiency on the pharmacokinetics of Orfadin has not been studied. | |||
|useInHepaticImpair=* The effect of hepatic dysfunction on the pharmacokinetics of nitisinone has not been studied. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Orfadin should be taken at least one hour before or at least two hours after a meal, since food effect is unknown. For young children, Orfadin capsules may be opened and the contents suspended in a small amount of water immediately before use. | |||
* Physicians should counsel patients and their parents or caregivers of the need to maintain dietary restriction of [[tyrosine]] and [[phenylalanine]] when taking Orfadin to treat [[hereditary tyrosinemia type 1]]. | |||
|monitoring=* Platelet and white blood cell counts should be monitored regularly during nitisinone therapy. | |||
* Regular monitoring for the hepatic complications by hepatic imaging ([[ultrasound]], [[computerized tomography]], [[magnetic resonance imaging]]) and laboratory tests, including serum alpha-fetoprotein concentration is recommended. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
===== | <!--Overdosage--> | ||
|overdose=* Accidental ingestion of Orfadin by individuals eating normal diets not restricted in [[tyrosine]] and [[phenylalanine]] will result in elevated tyrosine levels. In healthy volunteers given a single 1 mg/kg dose of nitisinone, the plasma [[tyrosine]] level reached a maximum of 1200 μmol/L from 48 to 120 hours after dosing. After a washout period of 14 days, the mean value of plasma [[tyrosine]] was still 808 μmol/L. Fasted follow-up samples obtained from volunteers several weeks later showed [[tyrosine]] values back to normal. There were no reports of changes in vital signs or laboratory data of any clinical significance. One patient reported sensitivity to sunlight. [[Hypertyrosinemia]] has been reported with Orfadin treatment | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 462261617 | |||
| IUPAC_name = 2-[2-nitro-4-(trifluoromethyl)benzoyl]<br>cyclohexane-1,3-dione | |||
| image = Nitisinone Wiki Str.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|CDI|nitisinone}} | |||
| licence_EU = Orfadin | |||
| licence_US = Nitisinone | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = Rx-only | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| elimination_half-life = Approximately 54 h | |||
===== | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 104206-65-7 | |||
| ATC_prefix = A16 | |||
| ATC_suffix = AX04 | |||
| PubChem = 115355 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00348 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 103195 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = K5BN214699 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D05177 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 50378 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1337 | |||
<!--Chemical data--> | |||
| C=14 | H=10 | F=3 | N=1 | O=5 | |||
| molecular_weight = 329.228 g/mol | |||
| smiles = O=C(c1ccc(cc1[N+]([O-])=O)C(F)(F)F)C2C(=O)CCCC2=O | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C14H10F3NO5/c15-14(16,17)7-4-5-8(9(6-7)18(22)23)13(21)12-10(19)2-1-3-11(12)20/h4-6,12H,1-3H2 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = OUBCNLGXQFSTLU-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction=* Nitisinone is a competitive inhibitor of 4-hydroxy-phenyl-pyruvate dioxygenase, an enzyme upstream of [[fumarylacetoacetate hydrolase]] (FAH) in the tyrosine catabolic pathway. By inhibiting the normal catabolism of tyrosine in patients with HT-1, nitisinone prevents the accumulation of the catabolic intermediates [[maleylacetoacetate]] and [[fumarylacetoacetate]]. | |||
* In patients with HT-1, these catabolic intermediates are converted to the toxic metabolites [[succinylacetone]] and [[succinylacetoacetate]], which are responsible for the observed liver and kidney toxicity. [[Succinylacetone]] can also inhibit the [[porphyrin]] synthesis pathway leading to the accumulation of [[5-aminolevulinate]], a [[neurotoxin]] responsible for the porphyric crises characteristic of [[HT-1]]. Nitisinone inhibits catabolism of the amino acid [[tyrosine]] and can result in elevated plasma levels of [[tyrosine]]. Therefore, treatment with nitisinone requires restriction of the dietary intake of [[tyrosine]] and [[phenylalanine]] to prevent the toxicity associated with elevated plasma levels of tyrosine | |||
= | <!--Structure--> | ||
|structure=* Orfadin contains nitisinone, which is a hydroxyphenyl-pyruvate dioxygenase inhibitor indicated as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of [[hereditary tyrosinemia type 1]] (HT-1). | |||
* Nitisinone occurs as white to yellowish-white, crystalline powder. It is practically insoluble in water, soluble in 2M sodium hydroxide and in methanol, and sparingly soluble in alcohol. | |||
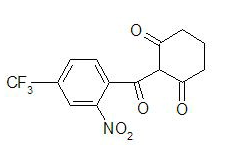
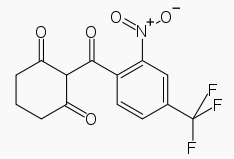
* Chemically, nitisinone is 2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione, and the structural formula is: | |||
: [[File:Nitisinone Str.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* Figure 1. The molecular formula is C14H10F3NO5 with a relative mass of 329.23 | |||
* Orfadin is a hard white-opaque capsule, marked as 2 mg, 5 mg or 10 mg strengths of nitisinone and is intended for oral administration. Each capsule contains 2 mg, 5 mg or 10 mg nitisinone, plus pre-gelatinized starch. The capsule shell is gelatin and titanium dioxide and the imprint is an iron oxide. | |||
= | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK=* Limited information exists on the metabolism, distribution, and excretion of nitisinone in rats. Nitisinone was greater than 90% bioavailable following oral administration of the labeled compound in rats and was distributed to different organs, particularly the liver and kidney, where radioactivity remained for 7 days after administration. Nitisinone was bio-transformed in rats and excreted via the urine. | |||
* No pharmacokinetic studies of nitisinone have been conducted in children or [[HT-1]] patients. | |||
* The single-dose pharmacokinetics of nitisinone have been studied in ten healthy male volunteers aged 19-39 years (median 32 years). Nitisinone, 1 mg/kg body weight, was administered as a capsule and a liquid. The median time for maximum plasma concentration was 3 hours for the capsule and 15 minutes for the liquid. The capsule and liquid formulation were found to be bioequivalent based on an analysis of area under the plasma concentration-time curve and maximum plasma concentration (Cmax). | |||
* No information on the metabolism of nitisinone in humans is available. The mean terminal plasma half-life of nitisinone in healthy male volunteers was 54 hours. The effect of food on the pharmacokinetics of nitisinone has not been studied. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
* No long-term studies in animals have been performed to evaluate the carcinogenic potential of nitisinone. Nitisinone was not genotoxic in the Ames test and the in vivo mouse liver unscheduled DNA synthesis (UDS) test. Nitisinone was mutagenic in the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test and in an in vivo mouse bone marrow micronucleus test. | |||
* In a single dose-group study in rats given 100 mg/kg (12 times the recommended clinical dose based on relative body surface area), reduced litter size, decreased pup weight at birth, and decreased survival of pups after birth was demonstrated. | |||
====ANIMAL TOXICOLOGY AND / OR PHARMACOLOGY==== | |||
* In pregnant mice given oral gavage doses of 5, 50, 250 mg/kg/day from gestation day 7 through 16, incomplete skeletal ossification of fetal bones was observed with doses of ≥5 mg/kg/day. In pregnant mice given the same doses from gestation day 7 through weaning, gestation length increased in animals given ≥50 mg/kg/day. Decreased pup survival by 9% compared to 5% in untreated controls was observed at 5 mg/kg/day. | |||
* In pregnant rabbits given oral gavage doses of 5, 12, 25 mg/kg/day from gestation day 7 through 19, maternal toxicity and incomplete skeletal ossification of fetal bones was observed with doses of ≥5 mg/kg/day. | |||
===== | <!--Clinical Studies--> | ||
|clinicalStudies======Clinical Studies in HT-1===== | |||
* The efficacy and safety of Orfadin in patients with [[HT-1]] were evaluated in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 21.7 years at enrollment (median age 9 months). | |||
* Patients were diagnosed with HT-1 by the presence of [[succinylacetone]] in the urine or plasma. All patients were treated with Orfadin at a starting dose of 0.6 to 1 mg/kg/day, and the dose was increased in some patients to 2 mg/kg/day based on weight, liver and kidney function tests, platelet count, serum amino acids, urinary [[phenolic acid]], plasma and urine [[succinylacetone]], erythrocyte PBG-synthase, and urine [[5-ALA]]. The median duration of treatment was 22 months (range less than 1 month to 80 months). Efficacy was assessed by comparison of survival and incidence of liver transplant to historical controls. | |||
* In this clinical study, for patients presenting with [[HT-1]] younger than 2 months of age who were treated with dietary restriction and nitisinone, 2- and 4-year survival probabilities were 88% and 88%, respectively. Data from historical controls showed that patients treated with dietary restriction alone had 2- and 4-year survival probabilities of 29% and 29%, respectively. For patients presenting between 2 and 6 months of age who were treated with dietary restrictions and nitisinone, 2- and 4-year survival probabilities were 94% and 94%, respectively. Data for historical controls showed that patients treated with dietary restriction alone had 2- and 4-year survival probabilities of 74% and 60%, respectively. | |||
* The effects on urine and plasma [[succinylacetone]], porphyrin metabolism, and urinary [[alpha-1-microglobulin]] were also assessed in this clinical study. | |||
* Urine succinylacetone was measured in 186 patients. In all 186 patients, urinary succinylacetone level decreased to less than 1 mmol/mol [[creatinine]]. The median time to normalization was 0.3 months. The probability of recurrence of abnormal values of urine succinylacetone was 1% at a nitisinone concentration of 37 μmol/L (95% confidence interval: 23-51 μmol/L). Plasma succinylacetone was measured in 172 patients. In 150 patients (87%), plasma succinylacetone decreased to less than 0.1 μmol/L. The median time to normalization was 3.9 months. | |||
* [[Porphyria-like crisis]] were reported in 3 patients (0.3% of cases per year) during the clinical study. This compares to an incidence of 5 to 20% of cases per year expected as part of the natural history of the disorder. An assessment of [[porphyria-like crises]] was performed because these events are commonly reported in patients with HT-1 who are not treated with nitisinone. | |||
* Urinary [[alpha-1-microglobulin]], a proposed marker of [[proximal tubular dysfunction]], was measured in 100 patients at baseline. | |||
* The overall median pretreatment level was 4.3 g/mol [[creatinine]]. After one year of treatment in a subgroup of patients (N=100), overall median alpha-1-microglobulin decreased by 1.5 g/mol [[creatinine]]. In patients 24 months of age and younger in whom multiple values were available (N=65), median alpha-1-microglobulin levels decreased from 5.0 to 3.0 g/mol [[creatinine]] (reference value for age ≤12 g/mol creatinine). In patients older than 24 months in whom multiple values were available (N=35), median alpha-1-microglobulin levels decreased from 2.8 to 2.0 g/mol [[creatinine]] (reference for age ≤6 g/mol creatinine). | |||
* The long term effect of nitisinone with regard to hepatic function in patients was not assessed. | |||
<!-- | <!--How Supplied--> | ||
| | |howSupplied=* Orfadin capsules are white and marked in black with ”NTBC” and identified as 2 mg, 5 mg or 10 mg strengths of nitisinone. The capsules are packed in a high density (HD) polyethylene container with a tamper-resistant low density (LD) polyethylene snap-on cap. Each bottle contains 60 capsules. | ||
: 2 mg white capsules imprinted "NTBC 2 mg" in black ink, NDC 66621-1002-6 | |||
| | : 5 mg white capsules imprinted "NTBC 5 mg" in black ink, NDC 66621-1005-6 | ||
| | : 10 mg white capsules imprinted "NTBC 10 mg" in black ink, NDC 66621-1010-6 | ||
|storage=* Store refrigerated, 2-8°C (36 - 46°F). | |||
|packLabel=====Ingredients and Appearance==== | |||
: [[File:Nitisinone Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=* Advise patients and caregivers of the need to maintain dietary restriction of tyrosine and phenylalanine when taking Orfadin to treat hereditary tyrosinemia type 1. | |||
* Advise patients and caregivers to report promptly unexplained eye symptoms, rash, jaundice, or excessive bleeding | |||
|fdaPatientInfo= | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* Orfadin®<ref>{{Cite web | title = Nitisinone | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1eecf439-77ff-40c8-aef8-71e024ea8b7c}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 16:48, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nitisinone is a Gastrointestinal Agent that is FDA approved for the treatment of hereditary tyrosinemia type 1 (HT-1). Common adverse reactions include hepatic neoplasm, liver failure, thrombocytopenia, leucopenia, visual system complaints including conjunctivitis, corneal opacity, keratitis, and photophobia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Orfadin(R) capsules (nitisinone) are indicated as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1
Dosage
- The recommended dose of Orfadin is 1 to 2 mg/kg divided into two daily doses. The initial dose is 1 mg/kg/day divided for morning and evening administration. Treatment with Orfadin should be initiated by a physician experienced in the treatment of HT-1.
- The dose of Orfadin may be adjusted in each patient. In patients whose erythrocyte PBG-synthase activity and urine 5-ALA and urine succinylacetone are not normalized within one month after the start of Orfadin treatment, the Orfadin dose may be increased to 1.5 mg/kg/day. In patients receiving 1.5 mg/kg/day, whose erythrocyte PBG-synthase activity and urine 5-ALA and urine succinylacetone remain elevated and whose plasma succinylacetone is not normalized after three months, the dose may be increased to up a maximum dose of 2 mg/kg/day.
- If plasma nitisinone concentration, plasma succinylacetone, urine 5-ALA and erythrocyte PBG-synthase activity are not available, clinical laboratory assessments should include urine succinyl acetone, liver function tests, alpha fetoprotein, and serum tyrosine and phenylalanine level. During initiation of therapy and during acute exacerbations, it may be necessary to follow more closely all available biochemical parameters
DOSAGE FORMS AND STRENGTHS
- Orfadin capsules (nitisinone) are available as white capsules imprinted with ”NTBC” followed by "2 mg", "5 mg", or "10 mg", indicating the actual amount of nitisinone in each capsule.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitisinone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitisinone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
- Orfadin(R) capsules (nitisinone) are indicated as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1 (HT-1)
Dosage
- The recommended dose of Orfadin is 1 to 2 mg/kg divided into two daily doses. The initial dose is 1 mg/kg/day divided for morning and evening administration. Treatment with Orfadin should be initiated by a physician experienced in the treatment of HT-1.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitisinone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitisinone in pediatric patients.
Contraindications
- None
Warnings
High Plasma Tyrosine Levels
- Orfadin is an inhibitor of 4-hydroxyphenyl-pyruvate dioxygenase, an enzyme in the tyrosine metabolic pathway. Therefore, treatment with Orfadin may cause an increase in blood tyrosine in patients with HT-1, and patients should maintain concomitant reduction in dietary tyrosine and phenylalanine while on Orfadin treatment. Plasma tyrosine levels should be maintained below 500 μmol/L. Inadequate restriction of tyrosine and phenylalanine intake may increase blood tyrosine levels and may be associated with blood tyrosine levels greater than 500 μmol/L leading to the following:
- Ocular signs and symptoms including corneal ulcers, corneal opacities, keratitis, conjunctivitis, eye pain, and photophobia have been reported in patients treated with Orfadin. Therefore, ophthalmologic examination including slit-lamp examination should be performed prior to initiating Orfadin treatment. Patients who develop photophobia, eye pain, or signs of inflammation such as redness, swelling, or burning of the eyes during treatment with Orfadin should undergo slit-lamp reexamination and immediate measurement of the plasma tyrosine concentration.
- Variable degrees of mental retardation and developmental delay. In patients treated with Orfadin who exhibit an abrupt change in neurologic status, a clinical laboratory assessment including plasma tyrosine levels should be performed.
- Painful hyperkeratotic plaques on the soles and palms.
- In patients with HT-1 treated with dietary restrictions and Orfadin who develop elevated plasma tyrosine levels, an assessment of dietary tyrosine intake should be performed.
LEUCOPENIA AND SEVERE THROMBOCYTOPENIA
- In clinical trials, patients treated with Orfadin and dietary restriction developed transient leucopenia (3%), thrombocytopenia (3%), or both (1.5%). One patient who developed both leucopenia and thrombocytopenia improved after the dose of Orfadin was decreased from 2 mg/kg to 1 mg/kg. No patients developed infections or bleeding as a result of the episodes of leucopenia and thromobocytopenia. Platelet and white blood cell counts should be monitored regularly during nitisinone therapy.
Adverse Reactions
Clinical Trials Experience
The following serious adverse reactions are described below and elsewhere in the labeling: High Plasma Tyrosine Levels.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
CLINICAL TRIALS EXPERIENCE
- Orfadin was studied in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 21.7 years at enrollment (median age 9 months), who were diagnosed with HT-1 by the presence of succinyl-acetone in the urine or plasma. The starting dose of Orfadin was 0.6 to 1 mg/kg/day, and the dose was increased in some patients to 2 mg/kg/day based on weight, biochemical, and enzyme markers. Median duration of treatment was 22.2 months (range 0.1 to 80 months).
- The most serious adverse reactions reported during Orfadin treatment were thrombocytopenia, leucopenia, porphyria, and ocular/visual complaints. Most patients with ocular/visual events had transient symptoms lasting less than one week, while 6 patients had symptoms lasting 16 to 672 days. Six patients had thrombocytopenia, with platelet counts 30,000/uL or lower in 3 patients. In 4 patients with thrombocytopenia, platelet counts returned to normal without change in Orfadin dose. In 2 patients platelet count returned to normal 2 weeks to 5 months after Orfadin treatment was discontinued. No patients developed infections or bleeding as a result of the episodes of leucopenia and thrombocytopenia.
- Other serious adverse events reported during Orfadin treatment were hepatic neoplasm, liver failure, and porphyric crises. Patients with hereditary tyrosinemia type 1 are at increased risk of developing porphyric crises, hepatic neoplasms, and liver failure requiring liver transplantation. These complications of HT-1 were observed in patients treated with nitisinone for a median of 22 months during the clinical trial (liver transplantation 13%, liver failure 7%, malignant hepatic neoplasms 5%, benign hepatic neoplasms 3%, porphyria 0.5%). Regular monitoring for these complications by hepatic imaging (ultrasound, computerized tomography, magnetic resonance imaging) and laboratory tests, including serum alpha-fetoprotein concentration is recommended. Patients with increasing alpha-fetoprotein levels or development of liver nodules during treatment with nitisinone should be evaluated for hepatic malignancy.
- The most common adverse reactions reported in the clinical trial are summarized in Table 1.
- Adverse reactions reported in less than 1% of the patients, regardless of causality assessment, included death, seizure, brain tumor, encephalopathy, headache, hyperkinesia, cyanosis, abdominal pain, diarrhea, enanthema, gastritis, gastroenteritis, gastrointestinal hemorrhage, melena, tooth discoloration, hepatic function disorder, elevated hepatic enzymes, liver enlargement, dehydration, hypoglycemia, thirst, infection, septicemia, otitis, infection (not otherwise specified), bronchitis, respiratory insufficiency, pathologic fracture, amenorrhea, nervousness, and somnolence.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nitisinone in the drug label.
Drug Interactions
There is limited information regarding Nitisinone Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in mice at oral doses of about 0.4, 4 and 20 times the recommended human dose (1 mg/kg/day) and in rabbits at oral doses of about 1.6, 4 and 8 times the recommended human dose based on the body surface area. In mice, nitisinone has been shown to cause incomplete skeletal ossification of fetal bones at 0.4, 4 and 20 times the recommended human dose, increased gestational length at 4 and 20 times the recommended human dose, and decreased pup survival at 0.4 times the recommended human dose based on the body surface area. In rabbits, nitisinone caused incomplete skeletal ossification of fetal bones at 1.6, 4 and 8 times the recommended human dose based on the body surface area.
- However, there are no adequate and well controlled studies in pregnant women. Nitisinone should be used during pregnancy only if the potential benefit justified the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitisinone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nitisinone during labor and delivery.
Nursing Mothers
- Although the exposure was not quantified naive pups that were exposed to Orfadin via breast milk showed signs of ocular toxicity and lower body weight. This suggests that Orfadin is excreted via breast milk in rats. It is not known whether Orfadin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Orfadin is administered to a nursing woman.
Pediatric Use
- Pediatric patients with HT-1, ages birth to 17 years have been treated with Orfadin in one open-label, uncontrolled clinical study. Monitoring of blood and urine succinyl acetone levels are recommended in the children to ensure adequate control. A nutritionist skilled in managing children with inborn errors of metabolism should be employed to design a low-protein diet deficient in tyrosine and phenylalanine.
Geriatic Use
- Clinical studies of nitisinone did not include any subjects aged 65 and over to determine whether they respond differently from younger subjects. HT-1 is presently a disease of the pediatric population. No pharmacokinetic studies of nitisinone have been performed in geriatric patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy in this patient population.
Gender
- The effect of gender on the pharmacokinetics of Orfadin has not been studied.
Race
- The effect of race on the pharmacokinetics of Orfadin has not been studied.
Renal Impairment
- The effect of renal insufficiency on the pharmacokinetics of Orfadin has not been studied.
Hepatic Impairment
- The effect of hepatic dysfunction on the pharmacokinetics of nitisinone has not been studied.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nitisinone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nitisinone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Orfadin should be taken at least one hour before or at least two hours after a meal, since food effect is unknown. For young children, Orfadin capsules may be opened and the contents suspended in a small amount of water immediately before use.
- Physicians should counsel patients and their parents or caregivers of the need to maintain dietary restriction of tyrosine and phenylalanine when taking Orfadin to treat hereditary tyrosinemia type 1.
Monitoring
- Platelet and white blood cell counts should be monitored regularly during nitisinone therapy.
- Regular monitoring for the hepatic complications by hepatic imaging (ultrasound, computerized tomography, magnetic resonance imaging) and laboratory tests, including serum alpha-fetoprotein concentration is recommended.
IV Compatibility
There is limited information regarding IV Compatibility of Nitisinone in the drug label.
Overdosage
- Accidental ingestion of Orfadin by individuals eating normal diets not restricted in tyrosine and phenylalanine will result in elevated tyrosine levels. In healthy volunteers given a single 1 mg/kg dose of nitisinone, the plasma tyrosine level reached a maximum of 1200 μmol/L from 48 to 120 hours after dosing. After a washout period of 14 days, the mean value of plasma tyrosine was still 808 μmol/L. Fasted follow-up samples obtained from volunteers several weeks later showed tyrosine values back to normal. There were no reports of changes in vital signs or laboratory data of any clinical significance. One patient reported sensitivity to sunlight. Hypertyrosinemia has been reported with Orfadin treatment
Pharmacology
| |
Nitisinone
| |
| Systematic (IUPAC) name | |
| 2-[2-nitro-4-(trifluoromethyl)benzoyl] cyclohexane-1,3-dione | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 329.228 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | Approximately 54 h |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Nitisinone is a competitive inhibitor of 4-hydroxy-phenyl-pyruvate dioxygenase, an enzyme upstream of fumarylacetoacetate hydrolase (FAH) in the tyrosine catabolic pathway. By inhibiting the normal catabolism of tyrosine in patients with HT-1, nitisinone prevents the accumulation of the catabolic intermediates maleylacetoacetate and fumarylacetoacetate.
- In patients with HT-1, these catabolic intermediates are converted to the toxic metabolites succinylacetone and succinylacetoacetate, which are responsible for the observed liver and kidney toxicity. Succinylacetone can also inhibit the porphyrin synthesis pathway leading to the accumulation of 5-aminolevulinate, a neurotoxin responsible for the porphyric crises characteristic of HT-1. Nitisinone inhibits catabolism of the amino acid tyrosine and can result in elevated plasma levels of tyrosine. Therefore, treatment with nitisinone requires restriction of the dietary intake of tyrosine and phenylalanine to prevent the toxicity associated with elevated plasma levels of tyrosine
Structure
- Orfadin contains nitisinone, which is a hydroxyphenyl-pyruvate dioxygenase inhibitor indicated as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1 (HT-1).
- Nitisinone occurs as white to yellowish-white, crystalline powder. It is practically insoluble in water, soluble in 2M sodium hydroxide and in methanol, and sparingly soluble in alcohol.
- Chemically, nitisinone is 2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione, and the structural formula is:
- Figure 1. The molecular formula is C14H10F3NO5 with a relative mass of 329.23
- Orfadin is a hard white-opaque capsule, marked as 2 mg, 5 mg or 10 mg strengths of nitisinone and is intended for oral administration. Each capsule contains 2 mg, 5 mg or 10 mg nitisinone, plus pre-gelatinized starch. The capsule shell is gelatin and titanium dioxide and the imprint is an iron oxide.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nitisinone in the drug label.
Pharmacokinetics
- Limited information exists on the metabolism, distribution, and excretion of nitisinone in rats. Nitisinone was greater than 90% bioavailable following oral administration of the labeled compound in rats and was distributed to different organs, particularly the liver and kidney, where radioactivity remained for 7 days after administration. Nitisinone was bio-transformed in rats and excreted via the urine.
- No pharmacokinetic studies of nitisinone have been conducted in children or HT-1 patients.
- The single-dose pharmacokinetics of nitisinone have been studied in ten healthy male volunteers aged 19-39 years (median 32 years). Nitisinone, 1 mg/kg body weight, was administered as a capsule and a liquid. The median time for maximum plasma concentration was 3 hours for the capsule and 15 minutes for the liquid. The capsule and liquid formulation were found to be bioequivalent based on an analysis of area under the plasma concentration-time curve and maximum plasma concentration (Cmax).
- No information on the metabolism of nitisinone in humans is available. The mean terminal plasma half-life of nitisinone in healthy male volunteers was 54 hours. The effect of food on the pharmacokinetics of nitisinone has not been studied.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No long-term studies in animals have been performed to evaluate the carcinogenic potential of nitisinone. Nitisinone was not genotoxic in the Ames test and the in vivo mouse liver unscheduled DNA synthesis (UDS) test. Nitisinone was mutagenic in the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test and in an in vivo mouse bone marrow micronucleus test.
- In a single dose-group study in rats given 100 mg/kg (12 times the recommended clinical dose based on relative body surface area), reduced litter size, decreased pup weight at birth, and decreased survival of pups after birth was demonstrated.
ANIMAL TOXICOLOGY AND / OR PHARMACOLOGY
- In pregnant mice given oral gavage doses of 5, 50, 250 mg/kg/day from gestation day 7 through 16, incomplete skeletal ossification of fetal bones was observed with doses of ≥5 mg/kg/day. In pregnant mice given the same doses from gestation day 7 through weaning, gestation length increased in animals given ≥50 mg/kg/day. Decreased pup survival by 9% compared to 5% in untreated controls was observed at 5 mg/kg/day.
- In pregnant rabbits given oral gavage doses of 5, 12, 25 mg/kg/day from gestation day 7 through 19, maternal toxicity and incomplete skeletal ossification of fetal bones was observed with doses of ≥5 mg/kg/day.
Clinical Studies
Clinical Studies in HT-1
- The efficacy and safety of Orfadin in patients with HT-1 were evaluated in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 21.7 years at enrollment (median age 9 months).
- Patients were diagnosed with HT-1 by the presence of succinylacetone in the urine or plasma. All patients were treated with Orfadin at a starting dose of 0.6 to 1 mg/kg/day, and the dose was increased in some patients to 2 mg/kg/day based on weight, liver and kidney function tests, platelet count, serum amino acids, urinary phenolic acid, plasma and urine succinylacetone, erythrocyte PBG-synthase, and urine 5-ALA. The median duration of treatment was 22 months (range less than 1 month to 80 months). Efficacy was assessed by comparison of survival and incidence of liver transplant to historical controls.
- In this clinical study, for patients presenting with HT-1 younger than 2 months of age who were treated with dietary restriction and nitisinone, 2- and 4-year survival probabilities were 88% and 88%, respectively. Data from historical controls showed that patients treated with dietary restriction alone had 2- and 4-year survival probabilities of 29% and 29%, respectively. For patients presenting between 2 and 6 months of age who were treated with dietary restrictions and nitisinone, 2- and 4-year survival probabilities were 94% and 94%, respectively. Data for historical controls showed that patients treated with dietary restriction alone had 2- and 4-year survival probabilities of 74% and 60%, respectively.
- The effects on urine and plasma succinylacetone, porphyrin metabolism, and urinary alpha-1-microglobulin were also assessed in this clinical study.
- Urine succinylacetone was measured in 186 patients. In all 186 patients, urinary succinylacetone level decreased to less than 1 mmol/mol creatinine. The median time to normalization was 0.3 months. The probability of recurrence of abnormal values of urine succinylacetone was 1% at a nitisinone concentration of 37 μmol/L (95% confidence interval: 23-51 μmol/L). Plasma succinylacetone was measured in 172 patients. In 150 patients (87%), plasma succinylacetone decreased to less than 0.1 μmol/L. The median time to normalization was 3.9 months.
- Porphyria-like crisis were reported in 3 patients (0.3% of cases per year) during the clinical study. This compares to an incidence of 5 to 20% of cases per year expected as part of the natural history of the disorder. An assessment of porphyria-like crises was performed because these events are commonly reported in patients with HT-1 who are not treated with nitisinone.
- Urinary alpha-1-microglobulin, a proposed marker of proximal tubular dysfunction, was measured in 100 patients at baseline.
- The overall median pretreatment level was 4.3 g/mol creatinine. After one year of treatment in a subgroup of patients (N=100), overall median alpha-1-microglobulin decreased by 1.5 g/mol creatinine. In patients 24 months of age and younger in whom multiple values were available (N=65), median alpha-1-microglobulin levels decreased from 5.0 to 3.0 g/mol creatinine (reference value for age ≤12 g/mol creatinine). In patients older than 24 months in whom multiple values were available (N=35), median alpha-1-microglobulin levels decreased from 2.8 to 2.0 g/mol creatinine (reference for age ≤6 g/mol creatinine).
- The long term effect of nitisinone with regard to hepatic function in patients was not assessed.
How Supplied
- Orfadin capsules are white and marked in black with ”NTBC” and identified as 2 mg, 5 mg or 10 mg strengths of nitisinone. The capsules are packed in a high density (HD) polyethylene container with a tamper-resistant low density (LD) polyethylene snap-on cap. Each bottle contains 60 capsules.
- 2 mg white capsules imprinted "NTBC 2 mg" in black ink, NDC 66621-1002-6
- 5 mg white capsules imprinted "NTBC 5 mg" in black ink, NDC 66621-1005-6
- 10 mg white capsules imprinted "NTBC 10 mg" in black ink, NDC 66621-1010-6
Storage
- Store refrigerated, 2-8°C (36 - 46°F).
Images
Drug Images
{{#ask: Page Name::Nitisinone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
Ingredients and Appearance
{{#ask: Label Page::Nitisinone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients and caregivers of the need to maintain dietary restriction of tyrosine and phenylalanine when taking Orfadin to treat hereditary tyrosinemia type 1.
- Advise patients and caregivers to report promptly unexplained eye symptoms, rash, jaundice, or excessive bleeding
Precautions with Alcohol
- Alcohol-Nitisinone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Orfadin®[1]
Look-Alike Drug Names
There is limited information regarding Nitisinone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.