Vismodegib: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= {{VP}} <!--Overview--> |genericName= |aOrAn= a |drugClass= hedgehog pathway inhibitor |indication= metastatic basal cell c...") |
(No difference)
|
Revision as of 17:45, 11 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Vismodegib is a hedgehog pathway inhibitor that is FDA approved for the {{{indicationType}}} of metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation. Common adverse reactions include muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgias, vomiting, and ageusia..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- ERIVEDGE capsule is indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates for surgery, and who are not candidates for radiation.
- The recommended dose of ERIVEDGE is 150 mg taken orally once daily until disease progression or until unacceptable toxicity.
- ERIVEDGE may be taken with or without food. Swallow capsules whole. Do not open or crush capsules.
- If a dose of ERIVEDGE is missed, do not make up that dose; resume dosing with the next scheduled dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Vismodegib in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vismodegib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Vismodegib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Vismodegib in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Vismodegib in pediatric patients.
Contraindications
- None.
Warnings
Precautions
- * Embryo-Fetal Death and Severe Birth Defects
- ERIVEDGE capsules can cause fetal harm when administered to a pregnant woman based on its mechanism of action. Vismodegib is teratogenic, embryotoxic, and fetotoxic in rats at maternal exposures lower than the human exposures at the recommended dose of 150 mg/day. In rats, malformations included craniofacial anomalies, open perineum, and absent or fused digits. Fetal retardations and variations were also observed.
- Verify pregnancy status prior to the initiation of ERIVEDGE. Advise male and female patients of the risks of embryo-fetal death and severe birth defects and the need for contraception during and after treatment. Advise patients to contact their healthcare provider immediately if they suspect they (or, for males, their female partner) may be pregnant. Female and male patients of reproductive potential should be counseled regarding pregnancy prevention and planning. If ERIVEDGE is used during pregnancy or if a patient becomes pregnant while taking (or for a male patient, if his female partner is exposed to) ERIVEDGE, the patient should be apprised of the potential hazard to the fetus. Report immediately exposure to ERIVEDGE during pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may have been exposed to ERIVEDGE during pregnancy, either directly or through seminal fluid, to participate in the ERIVEDGE pregnancy pharmacovigilance program by contacting the Genentech Adverse Event Line at 1-888-835-2555.
- Blood Donation
- Advise patients not to donate blood or blood products while receiving ERIVEDGE and for at least 7 months after the last dose of ERIVEDGE.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
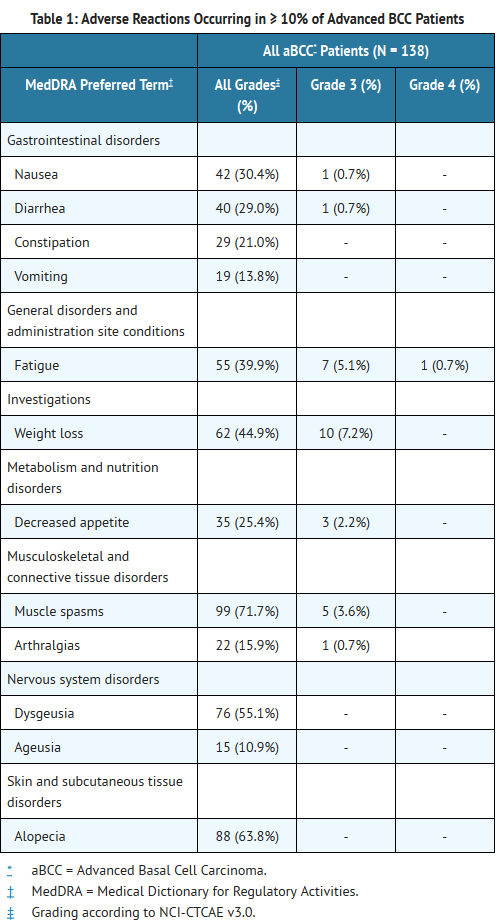
- ERIVEDGE capsule was administered as monotherapy at doses ≥ 150 mg orally daily in four open-label, uncontrolled, dose-ranging or fixed single dose clinical trials enrolling a total of 138 patients with advanced basal cell carcinoma (BCC). The median age of these patients was 61 years (range 21 to 101), 100% were White (including Hispanics), and 64% were male. The median duration of treatment was approximately 10 months (305 days; range 0.7 to 36 months); 111 patients received ERIVEDGE for 6 months or longer.
- The most common adverse reactions (≥ 10%) were muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, diarrhea, decreased appetite, constipation, arthralgias, vomiting, and ageusia (Table 1).
T1
- Amenorrhea:
- In clinical trials, a total of 3 of 10 pre-menopausal women developed amenorrhea while receiving ERIVEDGE [see Non-Clinical Toxicology (13.1)].
- Laboratory Abnormalities:
- Treatment-emergent Grade 3 laboratory abnormalities observed in clinical trials were hyponatremia in 6 patients (4%), hypokalemia in 2 patients (1%), and azotemia in 3 patients (2%).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Vismodegib in the drug label.
Drug Interactions
- Effects of Other Drugs on Vismodegib
- Drugs that Inhibit or Induce Drug Metabolizing Enzymes
- Vismodegib elimination involves multiple pathways. Vismodegib is predominantly excreted as an unchanged drug. Several minor metabolites are produced by multiple CYP enzymes. Although vismodegib is a substrate of CYP2C9 and CYP3A4 in vitro, CYP inhibition is not predicted to alter vismodegib systemic exposure since similar steady-state plasma vismodegib concentrations were observed in patients in clinical trials concomitantly treated with CYP3A4 inducers (i.e., carbamazepine, modafinil, phenobarbital) and those concomitantly treated with CYP3A4 inhibitors (i.e., erythromycin, fluconazole).
- Drugs that Inhibit Drug Transport Systems
- In vitro studies indicate that vismodegib is a substrate of the efflux transporter P-glycoprotein (P-gp). When ERIVEDGE is coadministered with drugs that inhibit P-gp (e.g. clarithromycin, erythromycin, azithromycin), systemic exposure of vismodegib and incidence of adverse events of ERIVEDGE may be increased.
- Drugs that Affect Gastric pH
- Drugs that alter the pH of the upper GI tract (e.g. proton pump inhibitors, H2-receptor antagonists, and antacids) may alter the solubility of vismodegib and reduce its bioavailability. However, no formal clinical study has been conducted to evaluate the effect of gastric pH altering agents on the systemic exposure of vismodegib. Increasing the dose of ERIVEDGE when coadministered with such agents is not likely to compensate for the loss of exposure. When ERIVEDGE is coadministered with a proton pump inhibitor, H2-receptor antagonist or antacid, systemic exposure of vismodegib may be decreased and the effect on efficacy of ERIVEDGE is unknown.
- Effects of Vismodegib on Other Drugs
- Results of a drug-drug interaction study conducted in cancer patients demonstrated that the systemic exposure of rosiglitazone (a CYP2C8 substrate) or oral contraceptives (ethinyl estradiol and norethindrone) is not altered when either drug is co-administered with vismodegib.
- In vitro studies indicate that vismodegib is an inhibitor of CYP2C8, CYP2C9, CYP2C19 and the transporter BCRP. Vismodegib does not induce CYP1A2, CYP2B6, or CYP3A4/5 in human hepatocytes.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- ERIVEDGE capsule can cause fetal harm when administered to a pregnant female based on its mechanism of action. Vismodegib is teratogenic in rats at doses corresponding to an exposure of 20% of the exposure at the recommended human dose (estimated AUC0-24hr steady-state exposure). In rats, malformations included craniofacial anomalies, open perineum, and absent or fused digits. Fetal retardations and variations were also observed. Vismodegib is embryolethal in rats at exposures within the range achieved at the recommended human dose. If ERIVEDGE is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the embryo or fetus. Report immediately exposure to ERIVEDGE during pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may have been exposed to ERIVEDGE during pregnancy, either directly or through seminal fluid, to participate in the ERIVEDGE pregnancy pharmacovigilance program by contacting the Genentech Adverse Event Line at 1-888-835-2555.
- In an embryo-fetal developmental toxicity study, pregnant rats were administered oral vismodegib at doses of 10, 60, or 300 mg/kg/day during the period of organogenesis. Pre- and post-implantation loss were increased at doses of ≥ 60 mg/kg/day (approximately ≥ 2 times the systemic exposure (AUC) in patients at the recommended human dose), which included early resorption of 100% of the fetuses. A dose of 10 mg/kg/day (approximately 0.2 times the AUC in patients at the recommended dose) resulted in malformations (including missing and/or fused digits, open perineum and craniofacial anomalies) and retardations or variations (including dilated renal pelvis, dilated ureter, and incompletely or unossified sternal elements, centra of vertebrae, or proximal phalanges and claws).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vismodegib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vismodegib during labor and delivery.
Nursing Mothers
- It is not known whether vismodegib is excreted in human breast milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from ERIVEDGE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of ERIVEDGE capsule have not been established in pediatric patients.
- In repeat-dose toxicology studies in rats, administration of oral vismodegib resulted in toxicities in bone and teeth. Effects on bone consisted of closure of the epiphyseal growth plate when oral vismodegib was administered for 26 weeks at ≥ 50 mg/kg/day (approximately ≥ 0.4 times the systemic exposure (AUC) in patients at the recommended human dose). Abnormalities in growing incisor teeth (including degeneration/necrosis of odontoblasts, formation of fluid-filled cysts in the dental pulp, ossification of the root canal, and hemorrhage resulting in breakage or loss of teeth) were observed after administration of oral vismodegib at ≥ 15 mg/kg/day (approximately ≥ 0.2 times the AUC in patients at the recommended human dose).
Geriatic Use
- Clinical studies of ERIVEDGE capsule did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
Gender
- ERIVEDGE capsule can cause harm to the embryo or fetus when administered during pregnancy. Counsel female and male patients regarding pregnancy prevention and planning. Advise patients to contact their healthcare provider immediately if they suspect they (or, for males, their female partner) may be pregnant [see Boxed Warning, Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
- Female patients
- Determine pregnancy status within 7 days prior to initiation of treatment in females of reproductive potential. For females with a negative pregnancy test, initiate a highly effective form of contraception (failure rate of less than 1%) prior to the first dose. Continue highly effective contraception during therapy and for 7 months after the last dose of ERIVEDGE. If a patient becomes pregnant while taking ERIVEDGE, or during the 7 months after the last dose of treatment, report the pregnancy to the Genentech Adverse Event Line at 1-888-835-2555. Encourage pregnant females to participate in the ERIVEDGE pregnancy pharmacovigilance program by calling the Genentech Adverse Event Line at 1-888-835-2555. Counsel pregnant females about the teratogenic risk to the fetus.
- Amenorrhea has been observed in clinical trials in females of reproductive potential. Reversibility of amenorrhea is unknown [see Adverse Reactions (6), Nonclinical Toxicology (13.1)].
- Male patients
- Male patients should use condoms with spermicide, even after a vasectomy, during sexual intercourse with female partners while being treated with ERIVEDGE capsule and for 2 months after the last dose to avoid exposing an embryo or fetus to vismodegib.
Race
There is no FDA guidance on the use of Vismodegib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Vismodegib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Vismodegib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Vismodegib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Vismodegib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Vismodegib in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Vismodegib in the drug label.
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Vismodegib in the drug label.
Pharmacology
There is limited information regarding Vismodegib Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Vismodegib in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Vismodegib in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Vismodegib in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Vismodegib in the drug label.
How Supplied
Storage
There is limited information regarding Vismodegib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Vismodegib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Vismodegib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Vismodegib in the drug label.
Precautions with Alcohol
- Alcohol-Vismodegib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Vismodegib |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Vismodegib |Label Name=Vismodegib11.png
}}
{{#subobject:
|Label Page=Vismodegib |Label Name=Vismodegib11.png
}}