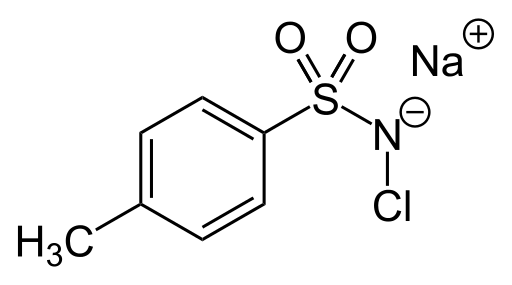
Tosylchloramide
| |
| |
| Names | |
|---|---|
| IUPAC name
N-chloro 4-methylbenzenesulfonamide, sodium salt
| |
| Other names
N-chloro para-toluenesulfonylamide,
sodium chloro[(4-methyl phenyl)sulfonyl]azanide, chloramine-T | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C7H7ClNO2S·Na (3H2O) | |
| Molar mass | 227.64 g/mol in usual tri-hydrated form 282 Daltons. |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
|
WikiDoc Resources for Tosylchloramide |
|
Articles |
|---|
|
Most recent articles on Tosylchloramide Most cited articles on Tosylchloramide |
|
Media |
|
Powerpoint slides on Tosylchloramide |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Tosylchloramide at Clinical Trials.gov Trial results on Tosylchloramide Clinical Trials on Tosylchloramide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Tosylchloramide NICE Guidance on Tosylchloramide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Tosylchloramide Discussion groups on Tosylchloramide Patient Handouts on Tosylchloramide Directions to Hospitals Treating Tosylchloramide Risk calculators and risk factors for Tosylchloramide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Tosylchloramide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Tosylchloramide or N-chloro tosylamide, sodium salt, sold as chloramine-T, is a N-chlorinated and N-deprotonated sulfonamide used as a biocide and a mild disinfectant. It is a white powder that gives unstable solutions with water. Trade names of chloramine-T products include Chloraseptin, Chlorazol, Clorina, Disifin, Halamid, Hydroclonazone, Trichlorol, Minachlor, and generic Chloramin T or Tosylchloramide Sodium, among others.
Uses
Iodination and radioiodination
Hypochlorite released from chloramine-T acts as an effective oxidizing agent for iodide to form iodine monochloride (ICl). ICl rapidly undergoes electrophilic substitution predominantly with activated aromatic rings, such as those of the amino acid tyrosine. Thus, chloramine-T is widely used for the incorporation of iodine to peptides and proteins. Chloramine-T together with iodogen or lactoperoxidase is commonly used for labeling peptides and proteins with radioiodine isotopes (123I, 125I or 131I).[1]
Biocide
Chloramine-T is available in tablet or powder form and has to be dissolved before use. It is sprayed on a surface and allowed to stand for at least 15 minutes before being wiped off or allowed to dry. It used in areas such as hospitals, laboratories, nursing homes, funeral homes, medical, dental and veterinary facilities, where control of pathogens is required, for disinfecting surfaces and soaking medical and dental equipment. The substance is also used for parasite control and for drinking water disinfection.
Chloramine-T is as an algicide, bactericide, virucide, fungicide (including spores), germicide. It is also effective against mycobacteria such as tuberculosis, foot-and-mouth disease and avian influenza. The molecular structure of toluenesulfonylamide is similar to para-aminobenzoic acid, an intermediate in bacterial metabolism, which is disrupted by this sulfonamide (in the same way as by a sulfa drug). Therefore, chloramine-T is capable of inhibiting with bacterial growth with two mechanisms, with the phenylsulfonamide moiety and the hypochlorite, which destroys the DNA structure via oxidation and thereby prevents microbes from reproducing and reforming.
Protective agent
Chloramine-T reacts readily with mustard gas to yield a harmless crystalline sulfimide; chloramine-T derivatives are being studied as protective agents against poison gas.[2]
Certifications
- EN 13713 Bactericidal
- EN 14675 Virucidal
- EN 14476 Virucidal Norovirus
- EN 1650 Fungicidal
- EN 13704 Sporicidal Clostridium difficile
References
- ↑ F.Rösch. Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences. Volume 4. Dordrecht/Boston/London: Kluwer Academic Publishers.
- ↑ Yasukazu Ura; Gozyo Sakata (2007), "Chloroamines", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 5
External links
- Chemicalland21.com: Chloramine T (Tosylchloramide sodium)
- InChem.org: Chloramine T
- "Disifin USA". Retrieved 2010-02-09.
- Pages with script errors
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with multiple CAS registry numbers
- Chemical articles with multiple PubChem CIDs
- Chemical articles with unknown parameter in Chembox
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed FDA identifier
- Articles with changed InChI identifier
- Chembox having DSD data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Antiseptics
- Pesticides
- Drug
- Sulfonamides
- Organochlorides
- Sodium compounds