Stereoisomerism
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Stereoisomers are isomeric molecules whose atomic connectivity is the same but whose atomic arrangement in space is different.
Enantiomers
Enantiomers are two stereoisomers that are related to each other by a reflection: they are mirror images of each other, which are non-superimposable. Every stereocenter in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different optical isomers of other compounds. For this reason, pure enantiomers exhibit the phenomenon of optical activity and can only be separated with the use of a chiral agent. In nature, only one enantiomer of most chiral biological compounds, such as amino acids, is present. As a result, different enantiomers of a compound may have substantially different biological effects.
Diastereomers
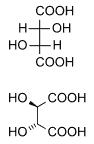
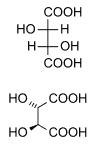
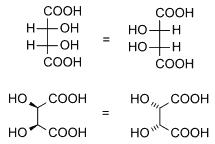
Diastereomers are stereoisomers not related through a reflection operation. They are not mirror images of each other. These include meso compounds, cis-trans (E-Z) isomers, and non-enantiomeric optical isomers. Diastereomers seldom have the same physical properties. In the example shown below, the meso form of tartaric acid forms a diastereomeric pair with both levo and dextro tartaric acids, which form an enantiomeric pair.
|
| |
|
(natural) tartaric acid |
D-(-)-tartaric acid |
mesotartaric acid |
|
(1:1) |
||
Cis-trans and E-Z isomerism
Stereoisomerism about double bonds arises because rotation about the double bond is restricted, keeping the substituents fixed relative to each other. If the substituents on either end of a double bond are the same, it is not considered a stereo bond.
Traditionally, double bond stereochemistry was described as either cis (Latin, on this side) or trans (Latin, across). (The terms cis and trans are also used to describe the relative position of two substituents on a ring; cis if on the same side, otherwise trans.) Due to occasional ambiguity, IUPAC adopted a more rigorous system wherein the substituents at each end of the double bond are assigned priority numbers. If the high priority substituents are on the same side of the bond it is assigned Z (Ger. zusammen, together). If they are on opposite sides it is E (Ger. entgegen, opposite).
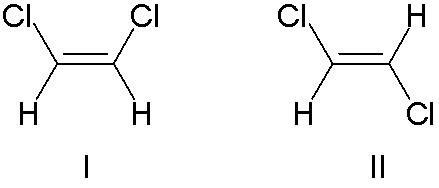
An example of double bond stereoisomerism is 1,2-dichloroethene, C2H2Cl2. Molecule I is Z-1,2-dichloroethene (chlorines on same side - the top) and molecule II (chlorines on opposite sides) is E-1,2-dichloroethene. There is no way of "superimposing" the structures on each other through bond rotation, because of the central double bond of C=C (composed of a sigma bond and a pi bond), through which rotation is not allowed. If rotation were allowed, such as a single bond would allow, these two molecules would be the same.
In contrast, for 1,2-dichloroethane, C2H4Cl2, which is similar except that it has an extra H attached to each C and a single bond, the E- and Z- forms do not exist. Since the carbon atoms can rotate around the single bond, in a flat projection of the molecule, all three atoms attached to one carbon could swap places and still represent the same structure.
Configurational isomers are diastereomers and can possess different physical, biological and chemical properties.
Conformers
Conformational isomerism is a form of isomerism that describes the phenomenon of molecules with the same structural formula having different shapes due to rotations about one or more bonds. Different conformations can have different energies, can usually interconvert, and are very rarely isolatable. For example, cyclohexane can exist in a variety of different conformations including a chair conformation and a boat conformation, but for cyclohexane itself, these can never be separated. The boat conformation represents an energy maximum (and not a transition state) on the conformational itinerary between the two equivalent chair forms. There are some molecules that can be isolated in several conformations, due to the large energy barriers between different conformations. 2,2,2',2'-Tetrasubstituted biphenyls can fit into this latter category.
References
- Compendium of Chemical Terminology, stereoisomerism
- Compendium of Chemical Terminology, geometric isomerism
- Columbia Encyclopedia. "Stereoisomers" in Encyclopedia.com, n.l., 2005, Link, December 7th 2005.
External links
- Chin. Chem. Soc., Vol. 46(3) 283 (1999) On the Assignment of Anomeric Configuration (pdf)
ar:تزامر فراغي da:Stereoisomer nl:Stereo-isomeer sr:Стереоизомерија fi:Stereoisomeria