Tosyl


A tosyl group (abbreviated Ts or Tos) combines the toluene and sulfonyl functional groups. The sulfonyl group consists of a hexavalent sulfur atom double bonded to two oxygen atoms and, in the tosylate group, an aromatic ring; an alkyl substituent on the R group forms a sulfonate ester. Thus, the tosylate group is the ester of toluenesulfonic acid. The para orientation illustrated (p-toluenesulfonyl) is most common, and by convention tosyl refers to the p-toluenesulfonate ester.
A tosylate ester has only a limited shelf life if it is stored outside of a desiccator as the free tosyl is readily hydrolysed by water in the presence of light. The tosyl group is electron-withdrawing. Hence, it is an excellent leaving group.
The tosyl group is also a protecting group for alcohols, prepared by combining the alcohol with toluenesulfonyl chloride in an aprotic solvent. Toluenesulfonyl chlorides activate alcohols for nucleophilic attack or elimination (dehydration).
Similarly, the brosyl (Bs) group or brosylate is a p-bromobenzenesulfonyl group with the methyl group of toluene replaced by a bromine atom. Nosyl groups in Nosylates (Ns) are 4-nitrobenzenesulfonyl groups with a nitro group in the para position.
Applications
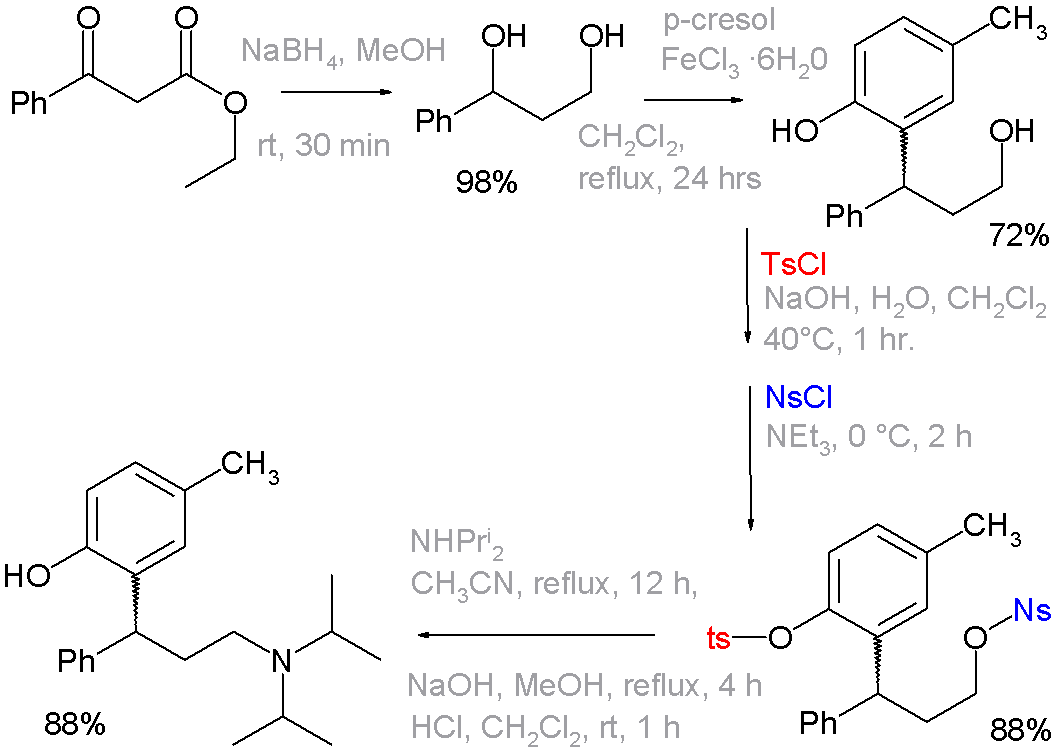
The use of these functional groups is examplified in an organic synthesis of the drug tolterodine, where in one of the steps a phenol group is blocked as a tosyl group and the primary alcohol as a nosyl group. The latter is a leaving group for displacement by diisopropylamine [1][2]:
References
- ↑ Kathlia A. De Castro, Jungnam Ko, Daejong Park, Sungdae Park, and Hakjune Rhee (2007). "Reduction of Ethyl Benzoylacetate and Selective Protection of 2-(3-Hydroxy-1-phenylpropyl)-4-methylphenol: A New and Facile Synthesis of Tolterodine". Organic Process Research & Development (ASAP article)
|format=requires|url=(help). doi:10.1021/op7001134. - ↑ Reaction sequence: organic reduction of ethyl benzoylacetate by sodium borohydride to a diol, followed by Friedel-Crafts alkylation with p-cresol and iron(III) chloride to a phenol. The tosyl and nosyl groupds are introduced as their respective chlorides with either sodium hydroxide or triethylamine as a base. The next step is nucleophilic displacement of the nosyl group by diisopropylamine, The remaining tosyl group is removed by another round of NaOH. Not shown: optical resolution by L-tartaric acid to optically pure (R)-isomer