Niobium(V) chloride
| Template:Chembox header | Niobium(V) chloride | |
|---|---|
 
| |
| Systematic name | Niobium(V) chloride Niobium pentachloride |
| Molecular formula | NbCl5 |
| Molar mass | 270.17 g/mol |
| Density | 2.75 g/cm3 |
| Solubility (water) | Decomposes |
| Solubility (dimethylformamide) | Good |
| Melting point | 204.7 °C |
| Boiling point | 254 °C |
| CAS number | [10026-12-7] |
| EINECS number | 233-059-8 |
| Template:Chembox header | Thermodynamic data | |
| Standard enthalpy of formation ΔfH°solid |
-797.47 kJ/mol |
| Standard molar entropy S°solid |
214.05 J.K−1.mol−1 |
| Template:Chembox header | Safety data | |
| EU classification | not listed |
| Flash point | non-flammable |
| RTECS number | QU0350000 |
| Template:Chembox header | Related compounds | |
| Related chlorides | Vanadium(IV) chloride Tantalum(V) chloride |
| Template:Chembox header |Disclaimer and references | |
Niobium(V) chloride, also known as niobium pentachloride, is a yellow crystalline solid often used as a starting material in niobium chemistry. It is prepared by heating niobium metal in chlorine. It is often contaminated with small amounts of niobium(V) oxychloride, NbOCl3, formed by hydrolysis or from traces of oxygen during the preparation. NbCl5 may be purified by sublimation.
Structure and properties
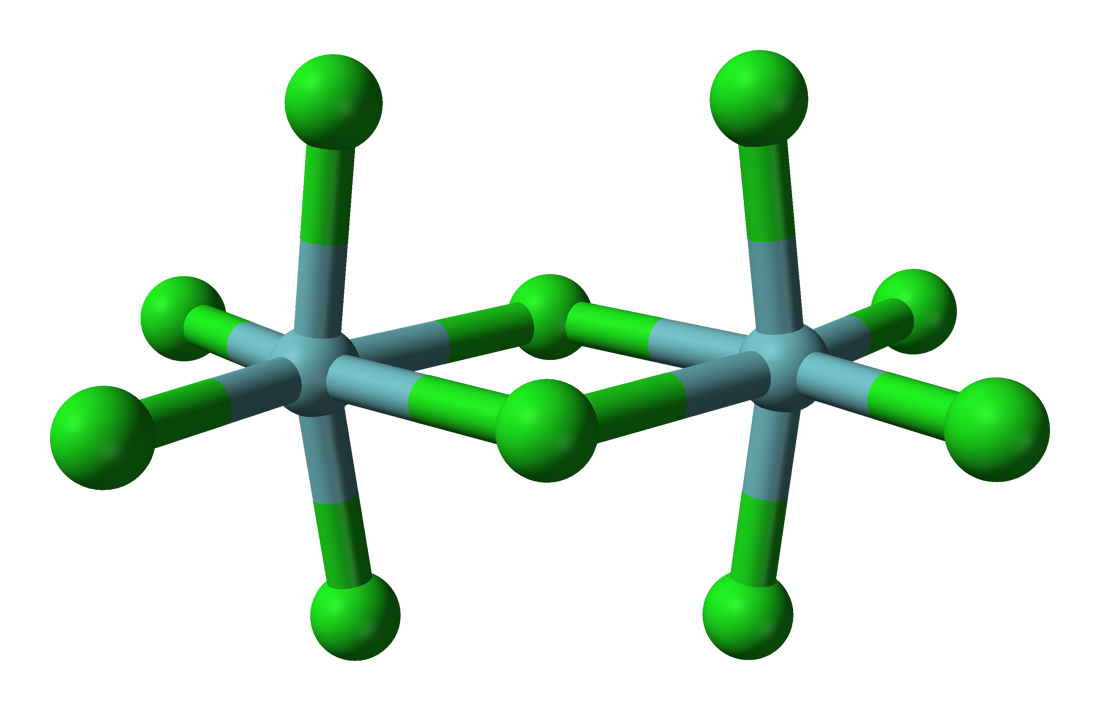
Niobium(V) chloride forms chloro-bridged dimers in the solid state (see figure). Each niobium centre is six-coordinate, but the octahedral coordination is significantly distorted. The niobium–chlorine bond lengths are 225 pm (terminal) and 256 pm (bridging), while the Nb–Cl–Nb angle at the bridge is 101.3°. The Nb–Nb distance is 395.1 pm, too long for any metal-metal interaction. NbBr5, TaCl5 and TaBr5 are isostructural with NbCl5, but NbI5 and TaI5 have different structures.
Uses
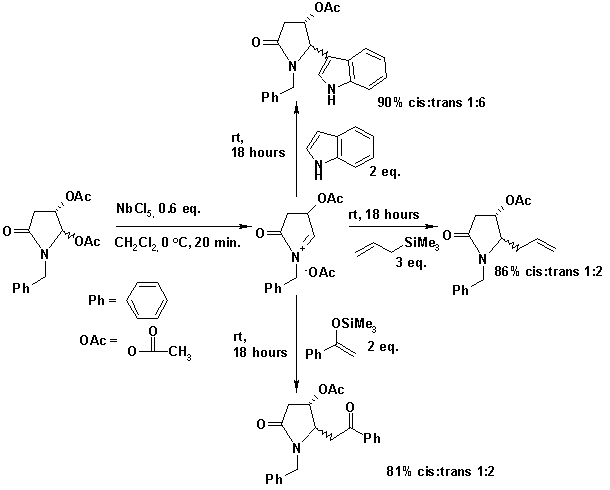
Niobium(V) chloride is used in organic chemistry as a Lewis acid in activating alkenes for the carbonyl-ene reaction and the Diels-Alder reaction. Niobium chloride can also generate N-acyliminium compounds from certain pyrrolidines which are substrates for nucleophiles such as allyltrimethylsilane, indole, or the silyl enol ether of benzophenone[1].
References
- ^ Andrade, C. K. Z.; Rocha, R. O.; Russowsky, D.; & Godoy, M. N. (2005). Studies on the Niobium Pentachloride-Mediated Nucleophilic Additions to an Enantiopure Cyclic N-acyliminium Ion Derived from (S)-malic acid. J. Braz. Chem. Soc. 16: 535–539. Online Article (.PDF file)