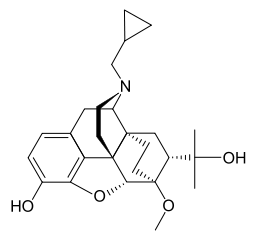
Diprenorphine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C26H35NO4 |
| Molar mass | 425.56 g/mol |
| 3D model (JSmol) | |
| |
|
WikiDoc Resources for Diprenorphine |
|
Articles |
|---|
|
Most recent articles on Diprenorphine Most cited articles on Diprenorphine |
|
Media |
|
Powerpoint slides on Diprenorphine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Diprenorphine at Clinical Trials.gov Trial results on Diprenorphine Clinical Trials on Diprenorphine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Diprenorphine NICE Guidance on Diprenorphine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Diprenorphine Discussion groups on Diprenorphine Patient Handouts on Diprenorphine Directions to Hospitals Treating Diprenorphine Risk calculators and risk factors for Diprenorphine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Diprenorphine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Diprenorphine (Revivon, M5050) is an opiate antagonist[1] used to reverse the effects of the super-potent opioid analgesics such as etorphine and carfentanil that are used for tranquilizing large animals in veterinary medicine.
Diprenorphine is the strongest opiate antagonist that is commercially available (some 100 times more potent as an antagonist than nalorphine),[2] and is used for reversing the effects of very strong opioids for which the binding affinity is so high that naloxone does not effectively or reliably reverse the narcotic effects.[3] These super-potent opioids are not used in humans because the dose for a human is so small that it would be difficult to measure properly, so there is an excessive risk of overdose leading to fatal respiratory depression. However conventional opioid derivatives are not strong enough to rapidly tranquilize large animals such as elephants and rhinos, so drugs such as etorphine or carfentanil are available for this purpose.
Diprenorphine is considered the specific antagonist for etorphine and carfentanil,[4] and is normally used to remobilise animals once veterinary procedures have been completed,[5] however it may also be used on humans in the event that they are accidentally exposed to etorphine or carfentanil, for instance if someone working with large animals suffered a needlestick injury with a dart from a tranquiliser dart gun that was loaded with etorphine or carfentanil, they would be given diprenorphine as an antidote.
In theory diprenorphine could also be used as an antidote for treating overdose of certain opioid derivatives which are used in humans, such as buprenorphine, for which the binding affinity is so high that naloxone does not reliably reverse the narcotic effects. However diprenorphine is not generally available in hospitals, instead a vial of diprenorphine is supplied with the etorphine or carfentanil specifically for reversing the effects of these drugs, so use of diprenorphine for treating buprenorphine overdose is not carried out in practice although it would work in theory.
References
- ↑ Lewis JW, Husbands SM. The orvinols and related opioids--high affinity ligands with diverse efficacy profiles. Current Pharmaceutical Design. 2004;10(7):717-32.
- ↑ Furst S, Hosztafi S, Friedmann T. Structure-Activity Relationships of Synthetic and Semisynthetic Opioid Agonists and Antagonists. Current Medicinal Chemistry, 1995; 1(6):423-440. ISSN:0929-8673
- ↑ Takemori AE, Hayashi G, Smits SE. Studies on the quantitative antagonism of analgesics by naloxone and diprenorphine. European Journal of Pharmacology. 1972 Oct;20(1):85-92.
- ↑ Jessup DA, Clark WE, Jones KR, Clark R, Lance WR. Immobilization of free-ranging desert bighorn sheep, tule elk, and wild horses, using carfentanil and xylazine: reversal with naloxone, diprenorphine, and yohimbine. Journal of the American Veterinary Medical Association. 1985 Dec 1;187(11):1253-4.
- ↑ Alford BT, Burkhart RL, Johnson WP. Etorphine and diprenorphine as immobilizing and reversing agents in captive and free-ranging mammals. Journal of the American Veterinary Medical Association. 1974 Apr 1;164(7):702-5.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Opioids
- Opioid antagonists
- Antidotes
- Drugs