Diisopropyl fluorophosphate
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
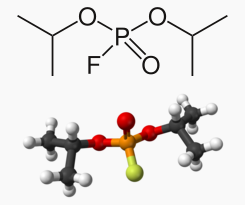
| Formula | C6H14FO3P |
| Molar mass | 184.146 g/mol |
| 3D model (JSmol) | |
| Melting point | −82 °C (−115.6 °F) |
| Boiling point | 46 °C (114.8 °F) 5 mmHg |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Diisopropyl fluorophosphate (DFP, diisopropyl phosphorofluoridate) is an oily, colorless or faint yellow liquid with the chemical formula C6H14FO3P. It is used in medicine and as an organophosphate insecticide. It is stable, but undergoes hydrolysis when subjected to moisture, producing hydrofluoric acid. It is a structural analog of sarin.
It is known also under names Demecarium, Difluorophate, Diflupyl, Diflurphate, Dyflos, Dyphlos, Fluoropropyl, Fluropryl, Fluostigmine, Humorsol, isofluorophate, isofluorphate, Neoglaucit, PF-3, PF3, T-1703, TL 466, and others.
Uses in medicine
Diisopropyl fluorophosphate (closely related to demecarium bromide) has been used in ophthalmology as a miotic agent in treatment of chronic glaucoma, as a miotic in veterinary medicine, and as an experimental agent in neuroscience because of its acetylcholinesterase inhibitory properties and ability to induce delayed peripheral neuropathy. It is known as fluostigmine and Dyflos in such uses.
Uses as toxin
The marked toxicity of esters of monofluorophosphoric acid was discovered in 1932, when Willy Lange and his PhD student Gerda von Krueger prepared the methyl, ethyl, n-propyl, and n-butyl esters and incidentally experienced their toxic effects. Another homologue of this series of esters, Diisopropyl fluorophosphate, was developed by British scientist Bernard Charles Saunders. On his search for compounds to be used as chemical warfare agents, Saunders was inspired by the report by Lange und Krueger and decided to prepare the new homologue which he labeled PF-3. It was much less deadly than tabun or sarin, however it could be mixed with mustard gas, forming a more potent mixture with significantly lower melting point, resulting in an agent suitable for use in cold weather. In military research, due to its physical and chemical similarities and comparatively low toxicity, it is used as a simulant of G-agents (GA, GB, GD, GF).
Diisopropyl fluorophosphate is a very potent neurotoxin. Its LD50 for rats is 1.3 mg/kg. It combines with the amino acid serine at the active site of the enzyme acetylcholinesterase, an enzyme that deactivates the neurotransmitter acetylcholine. Neurotransmitters are needed to continue the passage of nerve impulses from one neuron to another across the synapse. Once the impulse has been transmitted, acetylcholinesterase functions to deactivate the acetylcholine almost immediately by breaking it down. If the enzyme is inhibited, acetylcholine accumulates and nerve impulses cannot be stopped, causing prolonged muscle contraction. Paralysis occurs and death may result since the respiratory muscles are affected.
Sources
- Brenner, G. M. (2000): Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6
- Meiers, P. (2006): History of the fluorophosphates
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Drug
- Organophosphate insecticides
- Anticholinesterases
- Organophosphates
- Ophthalmology
- Neurotoxins