Cyclohexane conformation
Overview
Cyclohexane conformation is a much studied topic in organic chemistry because of the complex interrelationship between the different conformers of cyclohexane and its derivatives. Different conformers may have differing properties, including stability and chemical reactivity.
Historical background
The very first suggestion that cyclohexane may not be a flat molecule goes back a surprisingly long time. In 1890, Hermann Sachse, a 28-year-old assistant in Berlin, published instructions for folding a piece of paper to represent two forms of cyclohexane he called symmetrical and unsymmetrical (what we would now call chair and boat). He clearly understood that these forms had two positions for the hydrogens (again, to use modern terminology, axial and equatorial), that two chairs would probably interconvert, and even how certain substituents might favor one of the chair forms. Because he expressed all this in mathematical language, few chemists of the time understood his arguments. He had several goes at publishing these ideas, but none succeeded in capturing the imagination of chemists. His death in 1893 at the age of 31 meant his ideas sank into obscurity. It was only in 1918, when Ernst Mohr, using the then very new technique of x-ray crystallography, was able to determine the molecular structure of diamond, that it became recognised that Sachse's chair was the pivotal motif.[1][2][3]
Chair conformation
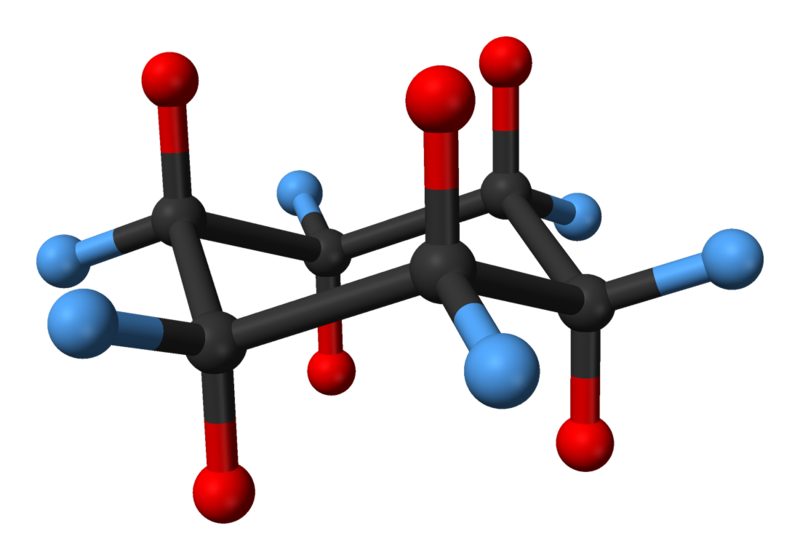
Due to the inherent tendency of the sp³ hybrid orbitals (and therefore the carbon-hydrogen bonds) on tetravalent carbons to form bond angles of 109.5 °, cyclohexane does not form a planar hexagonal arrangement with interior bond angles of 120 °. The chair conformation is a term used for the most stable chemical conformation of a six membered single bonded carbon ring like cyclohexane. Derek Barton and Odd Hassel both shared the Nobel Prize for work on the conformations of cyclohexane and various other molecules.
In the lowest-energy chair conformation, 6 of the 12 hydrogens are in axial positions (colored red)—their C-H bonds are parallel to each other and appear to stick up and down from the ring structure structure, the other 6 are in equatorial positions (colored blue)—they are splayed out around the perimeter of the ring. Note that in addition, one hydrogen at each position is "up" relative to the other being "down" at that position.
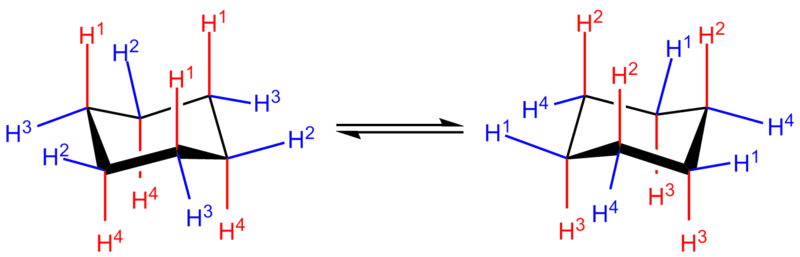
In stepping round the ring, it can be seen that the up-axial positions (H1 in the left-hand structure) alternate with up-equatorial positions (H2). Further, axial positions alternate sides around the ring (H1 axial-up vs H4 axial-down) and likewise the equatorial positions (H2 equatorial-up vs H3 equatorial-down). For two substituents attached to adjacent carbons on the ring as trans-1,2-cyclohexane, the substituents must either both be axial or both be equatorial to remain on opposite sides of the ring. Similarly, for cis-1,2-cyclohexane, one substituent must be axial and the other equatorial or vice versa. Various other substitution patterns are possible, each following the same patterns of relative geometric positioning.
In a process known as ring flipping or chair-flipping, the conformation of the ring changes, leading to the axial hydrogens becoming equatorial and the equatorial hydrogens becoming axial. However, the relative direction of the hydrogens to the ring remains the same: an "up" axial hydrogen, when flipped, remains an "up" equatorial hydrogen. The two chair conformations may differ in stability depending upon the identity of the functional groups. Generally, substituents are most stable when in equatorial positions, as in this case there are no 1,3-diaxial interaction between the axial substituent group and any other axial groups on the ring. For example, if there is a methyl group on carbon 1 in an axial position, it will interact with the axial hydrogens on carbon 3 and carbon 5. However, when there are electronegative heteroatoms involved, the opposite may be observed; this is called the anomeric effect. cyclohexane will be found in the chair formation 99.99% at 25 °C (i.e. 99.99% of all molecules in a solution sample will be in the chair formation).
Boat conformation
In addition to the chair conformation (1) with D3d symmetry cyclohexane can also exist in the half-chair or envelope (2), twist or twist-boat (3) with D2 symmetry and boat (4) conformers. Only the twist form is isolable as - like the chair form - it represents an energy minima. The boat conformation does not suffer from angle strain but has a higher energy than the chair form due to steric strain resulting from the two axial 1,4-hydrogen atoms. The torsional strain in the boat conformation has a maximum value because all the carbon bonds are eclipsed. Compare this to the chair with all bonds staggered and complete absence of torsional strain and the twist-boat with 2 out 6 bonds partially eclipsed. In the half-chair conformation 4 carbon atoms are located on a plane in which two bonds are fully eclipsed.
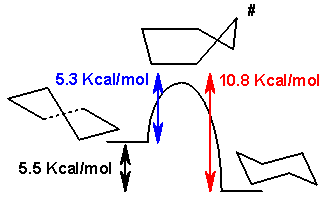
The boat and envelope forms are transition states between the twist forms and the twist and chair forms respectively, and are impossible to isolate. The twist-boat conformation is 5.5 kcal/mol (23 kJ/mol) less stable than the chair conformation. The energies of the two transition states are 6.6 kcal/mol (28 kJ/mol) (boat) and 10.8 kcal/mol (45 kJ/mol) (half chair) higher than that of the chair.[4] The ring flipping process can now be described with more precision as taking place through a twist-boat conformation and through two half-chair transition states.
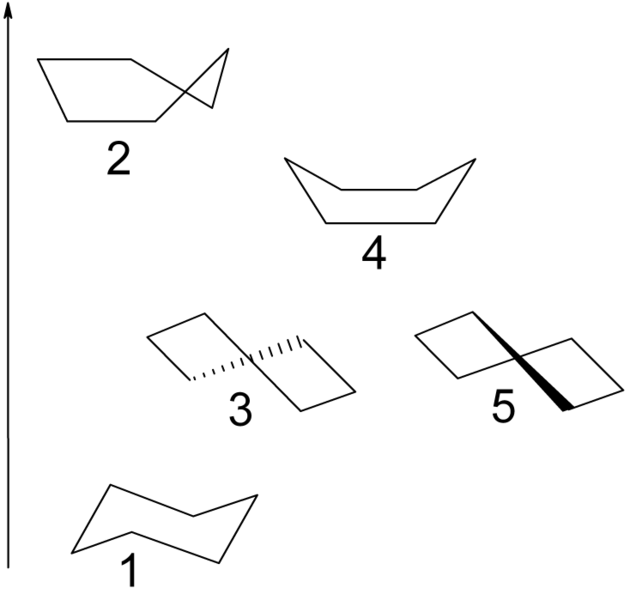
The difference in energy between the chair and the twist-boat conformation of cyclohexane can be measured indirectly by taking the difference in activation energy for the conversion of the chair to the twist-boat conformation and that of the reverse isomerization. The concentration of the twist-boat conformation at room temperature is very low (less than 0.1%) but at 1073 kelvins this concentration can reach 30%. The reverse reaction is measured by IR spectroscopy after rapidly cooling cyclohexane from 1073 K to 40 K, freezing in the large concentration of twist-boat conformation.
[6.6]Chiralane is a point group T molecule wholly composed of identical fused twist-boat cyclohexanes. Another view.
Cyclohexane derivatives
Substituents found on cyclohexane adopt cis and trans formations and cannot be easily switched by simple single sigma bond rotation as with linear molecules. Cis formation means that both substituents are found on the upper side of the 2 substituent placements on the carbon, while trans would mean that they were on opposing sides. Despite the fact that carbons on cyclohexane are linked by a single bond, the ring remains rigid, in that switching from cis to trans would require breaking the ring. The nomenclature for cis is dubbed (Z) while the name for trans is (E) to be placed in front of the IUPAC name.
For di-substituted cyclohexane rings (i.e. two groups on the ring), the relative orientation of the two substituents affect the energy of the possible conformations. For 1,2- and 1,4-di-substituted cyclohexane, a cis configuration leads to one axial and one equatorial group. This configuration can undergo chair flipping. For 1,2- and 1,4-di-substituted cyclohexane, a trans configuration leads to either both groups axial or both equatorial. In this case, the diaxial conformation is effectively prevented by its high steric strain (four gauche interactions more than the diequatorial). For 1,3-di-substituted cyclohexanes, the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups. Trans-1,3-di-substituted cyclohexanes are like cis-1,2- and cis-1,4- and can flip between the two equivalent axial/equatorial forms.
Derivatives of cyclohexane do exist that have a more stable twist-boat conformation. An example is 1,2,4,5-tetrathiane, an organosulfur compound with 4 methylene groups replaced by a sulfide group thus removing unfavorable 1,3-diaxial interactions. In the tetramethyl analogue 3,3,6,6-tetramethyl-1,2,4,5-tetrathiane the twist-boat conformation actually dominates. Also in cyclohexane-1,4-dione with the steric 1,4-hydrogen interaction removed, the actual stable conformation is the twist-boat.
Cis-1,4-di-tert-butylcyclohexane has an axial tert-butyl group in the chair conformation and conversion to the twist-boat conformation places both groups in more favorable equatorial positions. As a result the twist-boat conformation is more stable by 0.47 kcal/mol (1.96 kJ/mol) at 125 K as measured by NMR spectroscopy.
External links
References
- ↑ H. Sachse, Chem. Ber, 1890, 23, 1363; Z. Physik. Chem, 1892, 10, 203; Z. Physik. Chem., 1893, 11, 185-219.
- ↑ E. Mohr, J. Prakt. Chem., 1918, 98, 315 and Chem. Ber., 1922, 55, 230.
- ↑ This history is nicely summarised here.
- ↑ Conformational Study of cis-1,4-Di-tert-butylcyclohexane by Dynamic NMR Spectroscopy and Computational Methods. Observation of Chair and Twist-Boat Conformations Gill, G.; Pawar, D. M.; Noe, E. A J. Org. Chem. (Article); 2005; 70(26); 10726-10731. DOI: 10.1021/jo051654z Abstract
ar:تشكل مقعدي
de:Konformation#Konformationen_bei_zyklischen_Molek.C3.BClen