Alfentanil
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
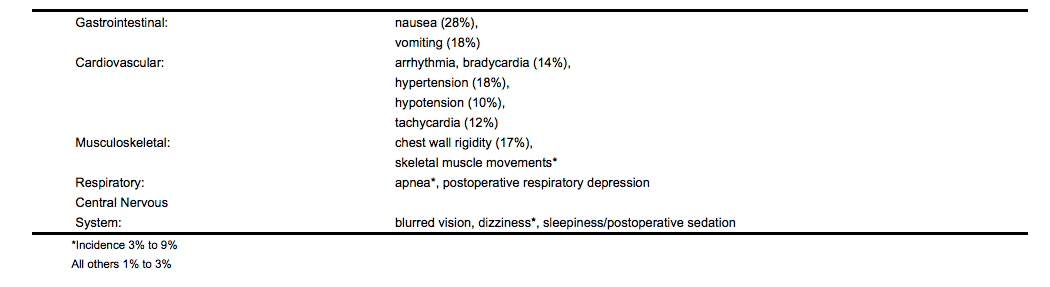
Alfentanil is an analgesic opioid that is FDA approved for the {{{indicationType}}} of adjunct surgical procedures and anesthesia. Common adverse reactions include cardiovascular: bradyarrhythmia (14% ), hypertension (18% ), hypotension (10% ), tachycardia (12% ), gastrointestinal: nausea (28% ), vomiting (18% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Administration of analgesic; Adjunct - Surgical procedure
- Surgery duration 30 min or less; 8-20 mcg/kg IV, then continuous IV infusion of 0.5-1 mcg/kg/min; total dose 8-40 mcg/kg
- Surgery duration 30-60 min; 20-50 mcg/kg IV, then maintenance IV injections of 5-15 mcg/kg every 5-20 min; total dose up to 75 mcg/kg
- Surgery duration longer than 45 min; 50-75 mcg/kg IV, then continuous IV infusion of 0.5-3 mcg/kg/min
- Anesthesia
- Individualized according to type and duration of surgical procedure/anesthesia
- When used as primary anesthetic agent; 130-245 mcg/kg induction dose followed by continuous IV infusion of 0.5-1.5 mcg/kg/min
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Alfentanil in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Alfentanil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Alfentanil is not recommended for children less than 12 years of age
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Alfentanil in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Alfentanil in pediatric patients.
Contraindications
Alfentanil is contraindicated in patients with known hypersensitivity to the drug or known intolerance to other opioid agonists.
Warnings
- Alfentanil should be administered only by persons specifically trained in the use of intravenous and general anesthetic agents and in the management of respiratory effects of potent opioids.
- An opioid antagonist, resuscitative and intubation equipment and oxygen should be readily available.
- Because of the possibility of delayed respiratory depression, monitoring of the patient must continue well after surgery.
- Alfentanil administered in initial dosages up to 20 mcg/kg may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is usually dose-related. Administration of alfentanil at anesthetic induction dosages (above 130 mcg/kg) will consistently produce muscular rigidity with an immediate onset. The onset of muscular rigidity occurs earlier than with other opioids. Alfentanil may produce muscular rigidity that involves all skeletal muscles, including those of the neck and extremities. The incidence may be reduced by: 1) routine methods of administration of neuromuscular blocking agents for balanced opioid anesthesia; 2) administration of up to 1/4 of the full paralyzing dose of a neuromuscular blocking agent just prior to administration of alfentanil at dosages up to 130 mcg/kg; following loss of consciousness, a full paralyzing dose of a neuromuscular blocking agent should be administered; or 3) simultaneous administration of alfentanil and a full paralyzing dose of a neuromuscular blocking agent when alfentanil is used in rapidly administered anesthetic dosages (above 130 mcg/kg).
- The neuromuscular blocking agent used should be appropriate for the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered alfentanil. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression.
- Patients receiving monitored anesthesia care (mac) should be continuously monitored by persons not involved in the conduct of the surgical or diagnostic procedure; oxygen supplementation should be immediately available and provided where clinically indicated; oxygen saturation should be continuously monitored; the patient should be observed for early signs of hypotension, apnea, upper airway obstruction and/or oxygen desaturation.
- Severe and unpredictable potentiation of monoamine oxidase (MAO) inhibitors has been reported for other opioid analgesics, and rarely with alfentanil. Therefore when alfentanil is administered to patients who have received MAO inhibitors within 14 days, appropriate monitoring and ready availability of vasodilators and betablockers for the treatment of hypertension is recommended.
Adverse Reactions
Clinical Trials Experience
- The most common adverse reactions of opioids are respiratory depression and skeletal muscle rigidity, particularly of the truncal muscles. Alfentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. See Clinical pharmacology, warnings, and precautions on the management of respiratory depression and skeletal muscle rigidity.
- The adverse experience profile from 696 patients receiving alfentanil for Monitored Anesthesia Care (MAC) is similar to the profile established with alfentanil during general anesthesia. Respiratory events reported during MAC included hypoxia, apnea, and bradypnea. Other adverse events reported by patients receiving alfentanil for MAC, in order of decreasing frequency, were nausea, hypotension, vomiting, pruritus, confusion, somnolence and agitation.
- The following adverse reaction information is derived from controlled and open clinical trials in 785 patients who received intravenous alfentanil during induction and maintenance of general anesthesia. The controlled trials included treatment comparisons with fentanyl, thiopental sodium, enflurane, saline placebo and halothane. The incidence of certain side effects is influenced by the type of use, e.g., chest wall rigidity has a higher reported incidence in clinical trials of alfentanil induction, and by the type of surgery, e.g., nausea and vomiting have a higher reported incidence in patients undergoing gynecologic surgery. The overall reports of nausea and vomiting with alfentanil were comparable to fentanyl.
Incidence Greater than 1% - Probably Causally Related (Derived from clinical trials)
Postmarketing Experience

Drug Interactions
There is limited information regarding Alfentanil Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Alfentanil has been shown to have an embryocidal effect in rats and rabbits when given in doses 2.5 times the upper human dose for a period of 10 days to over 30 days. These effects could have been due to maternal toxicity (decreased food consumption with increased mortality) following prolonged administration of the drug.
- No evidence of teratogenic effects has been observed after administration of alfentanil in rats or rabbits.
- There are no adequate and well-controlled studies in pregnant women. Alfentanil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Alfentanil in women who are pregnant.
Labor and Delivery
There are insufficient data to support the use of alfentanil in labor and delivery. Placental transfer of the drug has been reported; therefore, use in labor and delivery is not recommended.
Nursing Mothers
In one study of nine women undergoing postpartum tubal ligation, significant levels of alfentanil were detected in colostrum four hours after administration of 60 mcg/kg of alfentanil, with no detectable levels present after 28 hours. Caution should be exercised when alfentanil is administered to a nursing woman.
Pediatric Use
Adequate data to support the use of alfentanil in children under 12 years of age are not presently available.
Geriatic Use
There is no FDA guidance on the use of Alfentanil in geriatric settings.
Gender
There is no FDA guidance on the use of Alfentanil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Alfentanil with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Alfentanil in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Alfentanil in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Alfentanil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Alfentanil in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Alfentanil Administration in the drug label.
Monitoring
There is limited information regarding Alfentanil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Alfentanil and IV administrations.
Overdosage
- Overdosage would be manifested by extension of the pharmacological actions of alfentanil (see Clinical pharmacology) as with other potent opioid analgesics. No experience of overdosage with alfentanil was reported during clinical trials. The intravenous LD50 of alfentanil is 43 to 51 mg/kg in rats, 72 to 74 mg/kg in mice, 72 to 82 mg/kg in guinea pigs and 60 to 88 mg/kg in dogs. Intravenous administration of an opioid antagonist such as naloxone should be employed as a specific antidote to manage respiratory depression.
- The duration of respiratory depression following overdosage with alfentanil may be longer than the duration of action of the opioid antagonist. Administration of an opioid antagonist should not preclude immediate establishment of a patent airway, administration of oxygen, and assisted or controlled ventilation as indicated for hypoventilation or apnea. If respiratory depression is associated with muscular rigidity, a neuromuscular blocking agent may be required to facilitate assisted or controlled ventilation. Intravenous fluids and vasoactive agents may be required to manage hemodynamic instability.
Pharmacology
| |
Alfentanil
| |
| Systematic (IUPAC) name | |
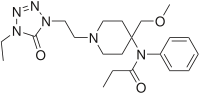
| N-{1-[2-(4-ethyl-5-oxo-4,5-dihydro-1H-1,2,3,4-tetrazol-1-yl)ethyl]-4-(methoxymethyl)piperidin-4-yl}-N-phenylpropanamide | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 416.517 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 140.8 °C (285 °F) |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 92% |
| Metabolism | Hepatic |
| Half life | 90–111 minutes |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Intravenous |
Mechanism of Action
There is limited information about the mechanism of action of Alfentanil.
Structure
- Alfentanil HCl Injection, USP is an opioid analgesic chemically designated as N-[1-[2-(4-ethyl-4,5-dihydro 5-oxo-1H-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4- piperidinyl]-N-phenylpropanamide monohydrochloride (1:1) with a molecular weight of 452.98 and an n-octanol:water partition coefficient of 128:1 at pH 7.4. The structural formula of Alfentanil hydrochloride is:

- Alfentanil HCl Injection, USP is a sterile, non-pyrogenic, preservative free aqueous solution containing alfentanil hydrochloride equivalent to 500 μg per mL of alfentanil base for intravenous injection. The solution, which contains sodium chloride for isotonicity, has a pH range of 4 to 6. Each mL contains: Active: Alfentanil base 500 mcg. Inactives: Sodium Chloride 9 mg and Water for Injection q.s.
Pharmacodynamics
There is limited information regarding Alfentanil Pharmacodynamics in the drug label.
Pharmacokinetics
- Alfentanil is an opioid analgesic with a rapid onset of action.
- At doses of 8 to 40 mcg/kg for surgical procedures lasting up to 30 minutes, alfentanil provides analgesic protection against hemodynamic responses to surgical stress with recovery times generally comparable to those seen with equipotent fentanyl dosages.
- For longer procedures, doses of up to 75 mcg/kg attenuate hemodynamic responses to laryngoscopy, intubation and incision, with recovery time comparable to fentanyl. At doses of 50 to 75 mcg/kg followed by a continuous infusion of 0.5 to 3 mcg/kg/min, alfentanil attenuates the catecholamine response with more rapid recovery and reduced need for postoperative analgesics as compared to patients administered enflurane. At doses of 5 mcg/kg, alfentanil provides analgesia for the conscious but sedated patient. Based on patient response, doses higher than 5 mcg/kg may be needed. Elderly or debilitated patients may require lower doses. High intrasubject and intersubject variability in the pharmacokinetic disposition of alfentanil has been reported.
- The pharmacokinetics of alfentanil can be described as a three-compartment model with sequential distribution half-lives of 1 and 14 minutes; and a terminal elimination half-life of 90 to 111 minutes (as compared to a terminal elimination half-life of approximately 475 minutes for fentanyl and approximately 265 minutes for sufentanil at doses of 250 mcg). The liver is the major site of biotransformation.
- Alfentanil has an apparent volume of distribution of 0.4 to 1 L/kg, which is approximately one-fourth to one-tenth that of fentanyl, with an average plasma clearance of 5 mL/kg/min as compared to approximately 8 mL/kg/min for fentanyl.
- Only 1% of the dose is excreted as unchanged drug; urinary excretion is the major route of elimination of metabolites. Plasma protein binding of alfentanil is approximately 92%.
- In one study involving 15 patients administered alfentanil with nitrous oxide/oxygen, a narrow range of plasma alfentanil concentrations, approximately 310 to 340 ng/mL, was shown to provide adequate anesthesia for intra-abdominal surgery, while lower concentrations, approximately 190 ng/mL, blocked responses to skin closure. Plasma concentrations between 100 to 200 ng/mL provided adequate anesthesia for superficial surgery.
- Alfentanil has an immediate onset of action. At dosages of approximately 105 mcg/kg, alfentanil produces hypnosis as determined by EEG patterns; an anesthetic ED90 of 182 mcg/kg for alfentanil in unpremedicated patients has been determined, based upon the ability to block response to placement of a nasopharyngeal airway. Based on clinical trials, induction dosage requirements range from 130 to 245 mcg/kg. For procedures lasting 30 to 60 minutes, loading dosages of up to 50 mcg/kg produce the hemodynamic response to endotracheal intubation and skin incision as comparable to those from fentanyl. A pre-intubation loading dose of 50 to 75 mcg/kg prior to a continuous infusion attenuates the response to laryngoscopy, intubation and incision. Subsequent administration of alfentanil infusion administered at a rate of 0.5 to 3 mcg/kg/min with nitrous oxide/oxygen attenuates sympathetic responses to surgical stress with more rapid recovery than enflurane.
- Requirements for volatile inhalation anesthetics were reduced by thirty to fifty percent during the first 60 minutes of maintenance in patients administered anesthetic doses (above 130 mcg/kg) of alfentanil as compared to patients given doses of 4 to 5 mg/kg thiopental for anesthetic induction. At anesthetic induction dosages, alfentanil provides a deep level of anesthesia during the first hour of anesthetic maintenance and provides attenuation of the hemodynamic response during intubation and incision.
- Following an anesthetic induction dose of alfentanil, requirements for alfentanil infusion are reduced by 30 to 50% for the first hour of maintenance.
- Patients with compromised liver function and those over 65 years of age have been found to have reduced plasma clearance and extended terminal elimination for alfentanil, which may prolong postoperative recovery. Repeated or continuous administration of alfentanil produces increasing plasma concentrations and an accumulation of the drug, particularly in patients with reduced plasma clearance.
- Bradycardia may be seen in patients administered alfentanil. The incidence and degree of bradycardia may be more pronounced when alfentanil is administered in conjunction with non-vagolytic neuromuscular blocking agents or in the absence of anticholinergic agents such as atropine.
- Administration of intravenous diazepam immediately prior to or following high doses of alfentanil has been shown to produce decreases in blood pressure that may be secondary to vasodilation; recovery may also be prolonged.
- Patients administered doses up to 200 mcg/kg of alfentanil have shown no significant increase in histamine levels and no clinical evidence of histamine release.
- Skeletal muscle rigidity is related to the dose and speed of administration of alfentanil. Muscular rigidity will occur with an immediate onset following anesthetic induction dosages. Preventative measures (see Warnings) may reduce the rate and severity.
- The duration and degree of respiratory depression and increased airway resistance usually increase with dose, but have also been observed at lower doses. Although higher doses may produce apnea and a longer duration of respiratory depression, apnea may also occur at low doses.
- During monitored anesthesia care (MAC), attention must be given to the respiratory effects of alfentanil. Decreased oxygen saturation, apnea, decreased respiratory rate, and upper airway obstruction can occur. (See Warnings)
Nonclinical Toxicology
There is limited information regarding Alfentanil Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Alfentanil Clinical Studies in the drug label.
How Supplied
- Alfentanil HCl Injection, USP for intravenous use. Each mL contains: Active: Alfentanil base 500 mcg. Inactives: Sodium Chloride 9 mg and WFI q.s. Alfentanil HCl Injection, USP is available as:
- NDC 17478-841-02, 2 mL Ampule in packages of 10
- NDC 17478-841-05, 5 mL Ampule in packages of 10
- NDC 17478-841-10, 10 mL Ampule in packages of 5
- NDC 17478-841-20, 20 mL Ampule in packages of 5
U.S. Patent No. 4,167,574 May 1995, November 1995 Premier Pro™ Rx Manufactured by: Akorn, Inc. Lake Forest, IL 60045 PREMIERPro™Rx is a trademark of Premier Inc., used under license. PAFA0N Rev. 04/13
Principal Display Panel Text for Container Label: NDC 17478-841-02 2 mL Ampule Alfentanil HCl Injection, USP CII 500 mcg/mL Alfentanil base Rx only May be habit forming. Premier Logo
Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Images
Drug Images
{{#ask: Page Name::Alfentanil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Alfentanil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Alfentanil Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Alfentanil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Alfentanil Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Alfentanil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Alfentanil |Label Name=Alfentanil label.png
}}