Urofollitropin: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} +, -{{EH}} +, -{{EJ}} +, -{{Editor Help}} +, -{{Editor Join}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag= | |||
{{VP}} | |||
<!--Overview--> | |||
{{ | |genericName= | ||
| | |||
| | |||
| | |||
| | |aOrAn= | ||
| | |||
| | a | ||
| | |||
| | |drugClass= | ||
| | |||
| | [[gonadotropin]] | ||
| | |||
| | |indication= | ||
| | |||
| | induction of [[ovulation]] in women who have previously received [[pituitary]] suppression and development of multiple follicles as part of an [[assisted reproductive technology]] (ART) cycle in [[ovulatory]] women who have previously received [[pituitary]] suppression | ||
| | |||
| | |hasBlackBoxWarning= | ||
| | |||
|adverseReactions= | |||
[[headache]], [[hot flashes]], [[OHSS]], [[pain]], [[respiratory disorder]], [[abdominal cramp]]s, abdominal fullness/enlargement, [[nausea]], [[pelvic pain]], and post retrieval pain | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Induction of Ovulation===== | |||
*The dosing scheme is stepwise and is individualized for each woman | |||
:*For women who have received GnRH agonist or antagonist pituitary suppression, a starting dose of 150 International Units per day of BRAVELLE® is administered subcutaneously or intramuscularly for 5 days in the first cycle of treatment. | |||
:*In subsequent cycles of treatment, the starting dose (and dosage adjustments) of BRAVELLE® should be determined based on the history of the ovarian response to BRAVELLE®. | |||
*The following should be considered when planning the woman's individualized dose of BRAVELLE®: | |||
:*Appropriate BRAVELLE® dose adjustment(s), based on clinical monitoring (including serum estradiol levels and vaginal ultrasound results), should be used to prevent multiple follicular growth and cycle cancellation. | |||
:*Do not make adjustments in dose more frequently than once every 2 days and do not exceed more than 75 to 150 International Units per adjustment. | |||
:*Use the lowest dose of BRAVELLE® that will achieve desired results. | |||
:*The maximum, individualized, daily dose of BRAVELLE® is 450 International Units per day. | |||
:*In general, do not exceed 12 days of treatment. | |||
:*When pre-ovulatory conditions are reached, administer human chorionic gonadotropin (hCG) to induce final oocyte maturation and ovulation. | |||
:*Withhold hCG in cases where the ovarian monitoring on the last day of BRAVELLE® treatment suggests an increased risk of ovarian hyperstimulation syndrome (OHSS). | |||
:*Encourage the woman and her partner to have intercourse daily, beginning on the day prior to the administration of hCG and until ovulation becomes apparent. | |||
:*Discourage intercourse when the risk for OHSS is increased. | |||
=====Assisted Reproduction Technology===== | |||
*The recommended dosing scheme for patients undergoing IVF follows a stepwise approach and is individualized for each woman. The recommended initial dose of BRAVELLE® for women who have received a GnRH agonist for pituitary suppression is 225 International Units. BRAVELLE® may be administered together with MENOPUR® (menotropins for injection, USP), and the total initial dose when the products are combined should not exceed 225 International Units (150 International Units of BRAVELLE® and 75 International Units of MENOPUR® or 75 International Units of BRAVELLE® and 150 International Units of MENOPUR®). | |||
:*Beginning on cycle day 2 or 3, a starting dose of 225 International Units of BRAVELLE® is administered subcutaneously daily until sufficient follicular development, as determined by ultrasound in combination with measurement of serum estradiol levels, is attained. In most cases, therapy should not exceed 12 days. | |||
:*Adjust the dose after 5 days based on the woman's ovarian response, as determined by ultrasound evaluation of follicular growth and serum estradiol levels. | |||
:*Do not make additional dosage adjustments more frequently than every 2 days or by more than 75 - 150 International Units at each adjustment. | |||
:*Continue treatment until adequate follicular development is evident, and then administer hCG. | |||
:*Withhold the administration of hCG in cases where the ovarian monitoring suggests an increased risk of OHSS on the last day of BRAVELLE® therapy. | |||
:*Do not administer daily doses of BRAVELLE® or BRAVELLE® in combination with MENOPUR® that exceed 450 International Units. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
* ConBRAVELLE® is contraindicated in women who exhibit: | |||
:*Prior [[hypersensitivity]] to BRAVELLE® or urofollitropins | |||
:*High levels of [[FSH]] indicating [[primary ovarian failure]] | |||
:*[[Pregnancy]] | |||
:*BRAVELLE® may cause fetal harm when administered to a pregnant woman. BRAVELLE® is contraindicated in women who are pregnant. If this drug is used during pregnancy, or if the woman becomes pregnant while taking this drug, the woman should be apprised of the potential hazard to a fetus. | |||
:*Presence of uncontrolled non-gonadal endocrinopathies (e.g., thyroid, adrenal, or pituitary disorders) | |||
:*Sex hormone dependent tumors of the reproductive tract and accessory organ | |||
:*Tumors of pituitary gland or hypothalamus | |||
:*[[Abnormal uterine bleeding]] of undetermined origin | |||
:*[[Ovarian cysts]] or enlargement of undetermined origin, not due to [[polycystic ovary syndrome]] | |||
<!--Warnings--> | |||
|warnings= | |||
====Precautions==== | |||
* Hypersensitivity and Anaphylactic Reactions | |||
:*Hypersensitivity/anaphylactic reactions associated with urofollitropins for injection, purified administration have been reported in some patients. These reactions presented as generalized urticaria, facial edema, angioneurotic edema, and/or dyspnea suggestive of laryngeal edema. The relationship of these symptoms to uncharacterized urinary proteins is uncertain. | |||
*Abnormal Ovarian Enlargement | |||
:*In order to minimize the hazard associated with abnormal ovarian enlargement that may occur with BRAVELLE® therapy, the lowest effective dose should be used [see Dosage and Administration (2.2, 2.3)]. Use of ultrasound monitoring of ovarian response and/or measurement of serum estradiol levels is important to minimize the risk of ovarian stimulation [see Warnings and Precautions (5.11)]. | |||
:*If the ovaries are abnormally enlarged on the last day of BRAVELLE® therapy, hCG should not be administered in order to reduce the chances of development of the Ovarian Hyperstimulation Syndrome [see Warnings and Precautions (5.3)]. Prohibit intercourse in women with significant ovarian enlargement because of the danger of hemoperitoneum resulting from rupture of ovarian cysts [see Warnings and Precautions (5.3)]. | |||
*Ovarian Hyperstimulation Syndrome (OHSS) | |||
:*OHSS: OHSS is a medical event distinct from uncomplicated ovarian enlargement. OHSS may progress rapidly to become a serious medical event. It is characterized by an apparent dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of development of OHSS are severe pelvic pain, nausea, vomiting, and weight gain. Abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria have been reported with OHSS. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic events [see Warnings and Precautions (5.4)]. Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with OHSS. | |||
:*OHSS occurs after treatment has been discontinued and reaches its maximum at about 7 to 10 days after treatment. Usually, OHSS resolves spontaneously with the onset of menses. If there is evidence that OHSS may be developing prior to hCG administration [see Warnings and Precautions (5.2)], the hCG must be withheld. | |||
:*Cases of OHSS are more common, more severe and more protracted if pregnancy occurs. OHSS develops rapidly; therefore patients should be followed for at least two weeks after hCG administration. | |||
:*If severe OHSS occurs, gonadotropins, including hCG, must be stopped and consideration should be given as to whether the woman needs be hospitalized. Treatment is primarily symptomatic and overall should consist of bed rest, fluid and electrolyte management, and analgesics (if needed). Because the use of diuretics can accentuate the diminished intravascular volume, diuretics should be avoided except in the late phase of resolution as described below. The management of OHSS may be divided into three phases as follows: | |||
*Acute Phase: | |||
:*Management should be directed at preventing hemoconcentration due to loss of intravascular volume to the third space and minimizing the risk of thromboembolic phenomena and kidney damage. Fluid intake and output, weight, hematocrit, serum and urinary electrolytes, urine specific gravity, BUN and creatinine, total proteins with albumin: globulin ratio, coagulation studies, electrocardiogram to monitor for hyperkalemia, and abdominal girth should be thoroughly assessed daily or more often based on the clinical need. Treatment, consisting of limited intravenous fluids, electrolytes, human serum albumin, is intended to normalize electrolytes while maintaining an acceptable but somewhat reduced intravascular volume. Full correction of the intravascular volume deficit may lead to an unacceptable increase in the amount of third space fluid accumulation. | |||
*Chronic Phase: | |||
:*After the acute phase is successfully managed as above, excessive fluid accumulation in the third space should be limited by instituting severe potassium, sodium, and fluid restriction. | |||
*Resolution Phase: | |||
:*As third space fluid returns to the intravascular compartment, a fall in hematocrit and increasing urinary output are observed in the absence of any increase in intake. Peripheral and/or pulmonary edema may result if the kidneys are unable to excrete third space fluid as rapidly as it is mobilized. Diuretics may be indicated during the resolution phase, if necessary, to combat pulmonary edema. | |||
*Do not remove ascitic, pleural, and pericardial fluid unless there is the necessity to relieve symptoms such as pulmonary distress or cardiac tamponade. | |||
*OHSS increases the risk of injury to the ovary. Pelvic examination or intercourse may cause rupture of an ovarian cyst, which may result in hemoperitoneum, and should be avoided. | |||
*If bleeding occurs and requires surgical intervention, the clinical objective should be to control the bleeding and retain as much ovarian tissue as possible. A physician experienced in the management of this syndrome, or who is experienced in the management of fluid and electrolyte imbalances, should be consulted. | |||
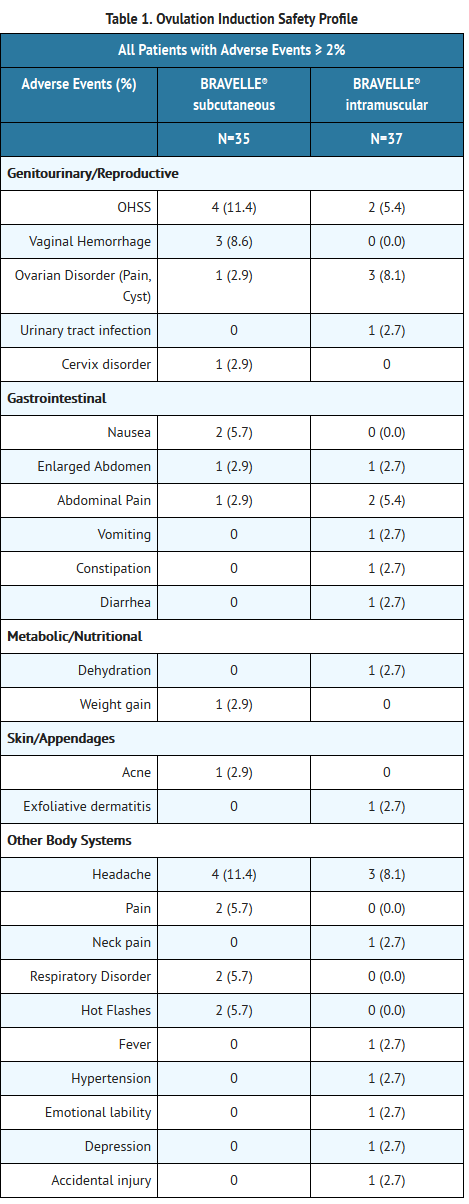
*In a clinical study of induction of ovulation indication, 6 of 72 (8.33%) BRAVELLE® treated women developed OHSS and 2 were classified as severe. In a clinical study for the development of multiple follicles as part of an IVF cycle, 3 of 60 women treated with BRAVELLE® developed OHSS and 1 was classified as severe. | |||
*Pulmonary and Vascular Complications | |||
:*Serious pulmonary conditions (e.g., atelectasis, acute respiratory distress syndrome) have been reported in women treated with gonadotropins. In addition, thromboembolic events both in association with, and separate from, the Ovarian Hyperstimulation Syndrome have been reported in women treated with gonadotropins. Intravascular thrombosis and embolism, which may originate in venous or arterial vessels, can result in reduced blood flow to critical organs or the extremities. Women with generally recognized risk factors for thrombosis, such as personal or family history, severe obesity, or thrombophilia, may have an increased risk of venous or arterial thromboembolic events during or following treatment with gonadotropins. Sequelae of such reactions have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb and rarely in myocardial infarctions. In rare cases, pulmonary complications and/or thromboembolic events have resulted in death. In women with recognized risk factors, the benefits of ovulation induction and assisted reproductive technology need to be weighed against the risks. Pregnancy also carries an increased risk of thrombosis. | |||
*Ovarian Torsion | |||
:*Ovarian torsion has been reported after treatment with gonadotropins. This may be related to OHSS, pregnancy, previous abdominal surgery, past history of ovarian torsion, previous or current ovarian cyst and polycystic ovaries. Damage to the ovary due to reduced blood supply can be limited by early diagnosis and immediate detorsion. | |||
* Multi-fetal Gestation and Birth | |||
:*Multi-fetal gestation and births have been reported with all gonadotropin therapy including therapy with BRAVELLE®. | |||
:*In a controlled study of 72 patients undergoing induction of ovulation, 66.7% of pregnancies of women treated with subcutaneous BRAVELLE® were multiples, while 28.6% of pregnancies in women treated with intramuscular BRAVELLE® were multiples. | |||
:*In a controlled study of 60 patients undergoing IVF, 34.8% of pregnancies of women treated with subcutaneous BRAVELLE® were multiples. | |||
:*Before beginning treatment with BRAVELLE®, advise the woman and her partner of the potential risk of multi-fetal gestation and birth. | |||
*Congenital Malformations | |||
:*The incidence of congenital malformations after some ART [specifically in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)] may be slightly higher than after spontaneous conception. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, maternal and paternal genetic background, sperm characteristics) and to the higher incidence of multi-fetal gestations after IVF or ICSI. There are no indications that the use of gonadotropins during IVF or ICSI is associated with an increased risk of congenital malformations. | |||
*Ectopic Pregnancy | |||
:*Since infertile women undergoing ART often have tubal abnormalities, the incidence of ectopic pregnancy may be increased. Early confirmation of intrauterine pregnancy should be determined by β-hCG testing and transvaginal ultrasound. | |||
*Spontaneous Abortion | |||
:*The risk of spontaneous abortion (miscarriage) is increased with gonadotropin products. However, causality has not been established. The increased risk may be a factor of the underlying infertility. | |||
*Ovarian Neoplasms | |||
:*There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have had multiple drug therapy for controlled ovarian stimulation; however, a causal relationship has not been established. | |||
*Laboratory Tests | |||
:*In most instances, treatment of women with BRAVELLE® will result only in follicular growth and maturation. In the absence of an endogenous LH surge, hCG is given when monitoring of the woman indicates that sufficient follicular development has occurred. This may be estimated by ultrasound alone or in combination with measurement of serum estradiol levels. The combination of both ultrasound and serum estradiol measurement are useful for monitoring follicular growth and maturation, timing of the ovulatory trigger, detecting ovarian enlargement and minimizing the risk of the Ovarian Hyperstimulation Syndrome and multiple gestation. | |||
:*The clinical confirmation of ovulation is obtained by direct or indirect indices of progesterone production as well as sonographic evidence of ovulation. | |||
*Direct or indirect indices of progesterone production: | |||
:*Urinary or serum luteinizing hormone (LH) rise | |||
:*A rise in basal body temperature | |||
:*Increase in serum progesterone | |||
:*Menstruation following the shift in basal body temperature | |||
*Sonographic evidence of ovulation: | |||
:*Collapsed follicle | |||
:*Fluid in the cul-de-sac | |||
:*Features consistent with corpus luteum formation | |||
:*Secretory endometrium | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
* Drug | |||
:* Description | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
* Intravenous | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
* Description | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
* Description | |||
====Management==== | |||
* Description | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* | |||
<!--Structure--> | |||
|structure= | |||
* | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | }} | ||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{ | {{LabelImage | ||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category: | [[Category:Drug]] | ||
Revision as of 14:21, 20 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Urofollitropin is a gonadotropin that is FDA approved for the {{{indicationType}}} of induction of ovulation in women who have previously received pituitary suppression and development of multiple follicles as part of an assisted reproductive technology (ART) cycle in ovulatory women who have previously received pituitary suppression. Common adverse reactions include headache, hot flashes, OHSS, pain, respiratory disorder, abdominal cramps, abdominal fullness/enlargement, nausea, pelvic pain, and post retrieval pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Induction of Ovulation
- The dosing scheme is stepwise and is individualized for each woman
- For women who have received GnRH agonist or antagonist pituitary suppression, a starting dose of 150 International Units per day of BRAVELLE® is administered subcutaneously or intramuscularly for 5 days in the first cycle of treatment.
- In subsequent cycles of treatment, the starting dose (and dosage adjustments) of BRAVELLE® should be determined based on the history of the ovarian response to BRAVELLE®.
- The following should be considered when planning the woman's individualized dose of BRAVELLE®:
- Appropriate BRAVELLE® dose adjustment(s), based on clinical monitoring (including serum estradiol levels and vaginal ultrasound results), should be used to prevent multiple follicular growth and cycle cancellation.
- Do not make adjustments in dose more frequently than once every 2 days and do not exceed more than 75 to 150 International Units per adjustment.
- Use the lowest dose of BRAVELLE® that will achieve desired results.
- The maximum, individualized, daily dose of BRAVELLE® is 450 International Units per day.
- In general, do not exceed 12 days of treatment.
- When pre-ovulatory conditions are reached, administer human chorionic gonadotropin (hCG) to induce final oocyte maturation and ovulation.
- Withhold hCG in cases where the ovarian monitoring on the last day of BRAVELLE® treatment suggests an increased risk of ovarian hyperstimulation syndrome (OHSS).
- Encourage the woman and her partner to have intercourse daily, beginning on the day prior to the administration of hCG and until ovulation becomes apparent.
- Discourage intercourse when the risk for OHSS is increased.
Assisted Reproduction Technology
- The recommended dosing scheme for patients undergoing IVF follows a stepwise approach and is individualized for each woman. The recommended initial dose of BRAVELLE® for women who have received a GnRH agonist for pituitary suppression is 225 International Units. BRAVELLE® may be administered together with MENOPUR® (menotropins for injection, USP), and the total initial dose when the products are combined should not exceed 225 International Units (150 International Units of BRAVELLE® and 75 International Units of MENOPUR® or 75 International Units of BRAVELLE® and 150 International Units of MENOPUR®).
- Beginning on cycle day 2 or 3, a starting dose of 225 International Units of BRAVELLE® is administered subcutaneously daily until sufficient follicular development, as determined by ultrasound in combination with measurement of serum estradiol levels, is attained. In most cases, therapy should not exceed 12 days.
- Adjust the dose after 5 days based on the woman's ovarian response, as determined by ultrasound evaluation of follicular growth and serum estradiol levels.
- Do not make additional dosage adjustments more frequently than every 2 days or by more than 75 - 150 International Units at each adjustment.
- Continue treatment until adequate follicular development is evident, and then administer hCG.
- Withhold the administration of hCG in cases where the ovarian monitoring suggests an increased risk of OHSS on the last day of BRAVELLE® therapy.
- Do not administer daily doses of BRAVELLE® or BRAVELLE® in combination with MENOPUR® that exceed 450 International Units.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Urofollitropin in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Urofollitropin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Urofollitropin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Urofollitropin in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Urofollitropin in pediatric patients.
Contraindications
- ConBRAVELLE® is contraindicated in women who exhibit:
- Prior hypersensitivity to BRAVELLE® or urofollitropins
- High levels of FSH indicating primary ovarian failure
- Pregnancy
- BRAVELLE® may cause fetal harm when administered to a pregnant woman. BRAVELLE® is contraindicated in women who are pregnant. If this drug is used during pregnancy, or if the woman becomes pregnant while taking this drug, the woman should be apprised of the potential hazard to a fetus.
- Presence of uncontrolled non-gonadal endocrinopathies (e.g., thyroid, adrenal, or pituitary disorders)
- Sex hormone dependent tumors of the reproductive tract and accessory organ
- Tumors of pituitary gland or hypothalamus
- Abnormal uterine bleeding of undetermined origin
- Ovarian cysts or enlargement of undetermined origin, not due to polycystic ovary syndrome
Warnings
Precautions
- Hypersensitivity and Anaphylactic Reactions
- Hypersensitivity/anaphylactic reactions associated with urofollitropins for injection, purified administration have been reported in some patients. These reactions presented as generalized urticaria, facial edema, angioneurotic edema, and/or dyspnea suggestive of laryngeal edema. The relationship of these symptoms to uncharacterized urinary proteins is uncertain.
- Abnormal Ovarian Enlargement
- In order to minimize the hazard associated with abnormal ovarian enlargement that may occur with BRAVELLE® therapy, the lowest effective dose should be used [see Dosage and Administration (2.2, 2.3)]. Use of ultrasound monitoring of ovarian response and/or measurement of serum estradiol levels is important to minimize the risk of ovarian stimulation [see Warnings and Precautions (5.11)].
- If the ovaries are abnormally enlarged on the last day of BRAVELLE® therapy, hCG should not be administered in order to reduce the chances of development of the Ovarian Hyperstimulation Syndrome [see Warnings and Precautions (5.3)]. Prohibit intercourse in women with significant ovarian enlargement because of the danger of hemoperitoneum resulting from rupture of ovarian cysts [see Warnings and Precautions (5.3)].
- Ovarian Hyperstimulation Syndrome (OHSS)
- OHSS: OHSS is a medical event distinct from uncomplicated ovarian enlargement. OHSS may progress rapidly to become a serious medical event. It is characterized by an apparent dramatic increase in vascular permeability, which can result in a rapid accumulation of fluid in the peritoneal cavity, thorax, and potentially, the pericardium. The early warning signs of development of OHSS are severe pelvic pain, nausea, vomiting, and weight gain. Abdominal pain, abdominal distension, gastrointestinal symptoms including nausea, vomiting and diarrhea, severe ovarian enlargement, weight gain, dyspnea, and oliguria have been reported with OHSS. Clinical evaluation may reveal hypovolemia, hemoconcentration, electrolyte imbalances, ascites, hemoperitoneum, pleural effusions, hydrothorax, acute pulmonary distress, and thromboembolic events [see Warnings and Precautions (5.4)]. Transient liver function test abnormalities suggestive of hepatic dysfunction, which may be accompanied by morphologic changes on liver biopsy, have been reported in association with OHSS.
- OHSS occurs after treatment has been discontinued and reaches its maximum at about 7 to 10 days after treatment. Usually, OHSS resolves spontaneously with the onset of menses. If there is evidence that OHSS may be developing prior to hCG administration [see Warnings and Precautions (5.2)], the hCG must be withheld.
- Cases of OHSS are more common, more severe and more protracted if pregnancy occurs. OHSS develops rapidly; therefore patients should be followed for at least two weeks after hCG administration.
- If severe OHSS occurs, gonadotropins, including hCG, must be stopped and consideration should be given as to whether the woman needs be hospitalized. Treatment is primarily symptomatic and overall should consist of bed rest, fluid and electrolyte management, and analgesics (if needed). Because the use of diuretics can accentuate the diminished intravascular volume, diuretics should be avoided except in the late phase of resolution as described below. The management of OHSS may be divided into three phases as follows:
- Acute Phase:
- Management should be directed at preventing hemoconcentration due to loss of intravascular volume to the third space and minimizing the risk of thromboembolic phenomena and kidney damage. Fluid intake and output, weight, hematocrit, serum and urinary electrolytes, urine specific gravity, BUN and creatinine, total proteins with albumin: globulin ratio, coagulation studies, electrocardiogram to monitor for hyperkalemia, and abdominal girth should be thoroughly assessed daily or more often based on the clinical need. Treatment, consisting of limited intravenous fluids, electrolytes, human serum albumin, is intended to normalize electrolytes while maintaining an acceptable but somewhat reduced intravascular volume. Full correction of the intravascular volume deficit may lead to an unacceptable increase in the amount of third space fluid accumulation.
- Chronic Phase:
- After the acute phase is successfully managed as above, excessive fluid accumulation in the third space should be limited by instituting severe potassium, sodium, and fluid restriction.
- Resolution Phase:
- As third space fluid returns to the intravascular compartment, a fall in hematocrit and increasing urinary output are observed in the absence of any increase in intake. Peripheral and/or pulmonary edema may result if the kidneys are unable to excrete third space fluid as rapidly as it is mobilized. Diuretics may be indicated during the resolution phase, if necessary, to combat pulmonary edema.
- Do not remove ascitic, pleural, and pericardial fluid unless there is the necessity to relieve symptoms such as pulmonary distress or cardiac tamponade.
- OHSS increases the risk of injury to the ovary. Pelvic examination or intercourse may cause rupture of an ovarian cyst, which may result in hemoperitoneum, and should be avoided.
- If bleeding occurs and requires surgical intervention, the clinical objective should be to control the bleeding and retain as much ovarian tissue as possible. A physician experienced in the management of this syndrome, or who is experienced in the management of fluid and electrolyte imbalances, should be consulted.
- In a clinical study of induction of ovulation indication, 6 of 72 (8.33%) BRAVELLE® treated women developed OHSS and 2 were classified as severe. In a clinical study for the development of multiple follicles as part of an IVF cycle, 3 of 60 women treated with BRAVELLE® developed OHSS and 1 was classified as severe.
- Pulmonary and Vascular Complications
- Serious pulmonary conditions (e.g., atelectasis, acute respiratory distress syndrome) have been reported in women treated with gonadotropins. In addition, thromboembolic events both in association with, and separate from, the Ovarian Hyperstimulation Syndrome have been reported in women treated with gonadotropins. Intravascular thrombosis and embolism, which may originate in venous or arterial vessels, can result in reduced blood flow to critical organs or the extremities. Women with generally recognized risk factors for thrombosis, such as personal or family history, severe obesity, or thrombophilia, may have an increased risk of venous or arterial thromboembolic events during or following treatment with gonadotropins. Sequelae of such reactions have included venous thrombophlebitis, pulmonary embolism, pulmonary infarction, cerebral vascular occlusion (stroke), and arterial occlusion resulting in loss of limb and rarely in myocardial infarctions. In rare cases, pulmonary complications and/or thromboembolic events have resulted in death. In women with recognized risk factors, the benefits of ovulation induction and assisted reproductive technology need to be weighed against the risks. Pregnancy also carries an increased risk of thrombosis.
- Ovarian Torsion
- Ovarian torsion has been reported after treatment with gonadotropins. This may be related to OHSS, pregnancy, previous abdominal surgery, past history of ovarian torsion, previous or current ovarian cyst and polycystic ovaries. Damage to the ovary due to reduced blood supply can be limited by early diagnosis and immediate detorsion.
- Multi-fetal Gestation and Birth
- Multi-fetal gestation and births have been reported with all gonadotropin therapy including therapy with BRAVELLE®.
- In a controlled study of 72 patients undergoing induction of ovulation, 66.7% of pregnancies of women treated with subcutaneous BRAVELLE® were multiples, while 28.6% of pregnancies in women treated with intramuscular BRAVELLE® were multiples.
- In a controlled study of 60 patients undergoing IVF, 34.8% of pregnancies of women treated with subcutaneous BRAVELLE® were multiples.
- Before beginning treatment with BRAVELLE®, advise the woman and her partner of the potential risk of multi-fetal gestation and birth.
- Congenital Malformations
- The incidence of congenital malformations after some ART [specifically in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI)] may be slightly higher than after spontaneous conception. This slightly higher incidence is thought to be related to differences in parental characteristics (e.g., maternal age, maternal and paternal genetic background, sperm characteristics) and to the higher incidence of multi-fetal gestations after IVF or ICSI. There are no indications that the use of gonadotropins during IVF or ICSI is associated with an increased risk of congenital malformations.
- Ectopic Pregnancy
- Since infertile women undergoing ART often have tubal abnormalities, the incidence of ectopic pregnancy may be increased. Early confirmation of intrauterine pregnancy should be determined by β-hCG testing and transvaginal ultrasound.
- Spontaneous Abortion
- The risk of spontaneous abortion (miscarriage) is increased with gonadotropin products. However, causality has not been established. The increased risk may be a factor of the underlying infertility.
- Ovarian Neoplasms
- There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have had multiple drug therapy for controlled ovarian stimulation; however, a causal relationship has not been established.
- Laboratory Tests
- In most instances, treatment of women with BRAVELLE® will result only in follicular growth and maturation. In the absence of an endogenous LH surge, hCG is given when monitoring of the woman indicates that sufficient follicular development has occurred. This may be estimated by ultrasound alone or in combination with measurement of serum estradiol levels. The combination of both ultrasound and serum estradiol measurement are useful for monitoring follicular growth and maturation, timing of the ovulatory trigger, detecting ovarian enlargement and minimizing the risk of the Ovarian Hyperstimulation Syndrome and multiple gestation.
- The clinical confirmation of ovulation is obtained by direct or indirect indices of progesterone production as well as sonographic evidence of ovulation.
- Direct or indirect indices of progesterone production:
- Urinary or serum luteinizing hormone (LH) rise
- A rise in basal body temperature
- Increase in serum progesterone
- Menstruation following the shift in basal body temperature
- Sonographic evidence of ovulation:
- Collapsed follicle
- Fluid in the cul-de-sac
- Features consistent with corpus luteum formation
- Secretory endometrium
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Urofollitropin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Urofollitropin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Urofollitropin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Urofollitropin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Urofollitropin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Urofollitropin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Urofollitropin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Urofollitropin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Urofollitropin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Urofollitropin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Urofollitropin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Urofollitropin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Urofollitropin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Urofollitropin in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Urofollitropin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Urofollitropin in the drug label.
Pharmacology
There is limited information regarding Urofollitropin Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Urofollitropin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Urofollitropin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Urofollitropin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Urofollitropin in the drug label.
How Supplied
Storage
There is limited information regarding Urofollitropin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Urofollitropin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Urofollitropin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Urofollitropin in the drug label.
Precautions with Alcohol
- Alcohol-Urofollitropin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Urofollitropin |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Urofollitropin |Label Name=Urofollitropin11.png
}}
{{#subobject:
|Label Page=Urofollitropin |Label Name=Urofollitropin11.png
}}